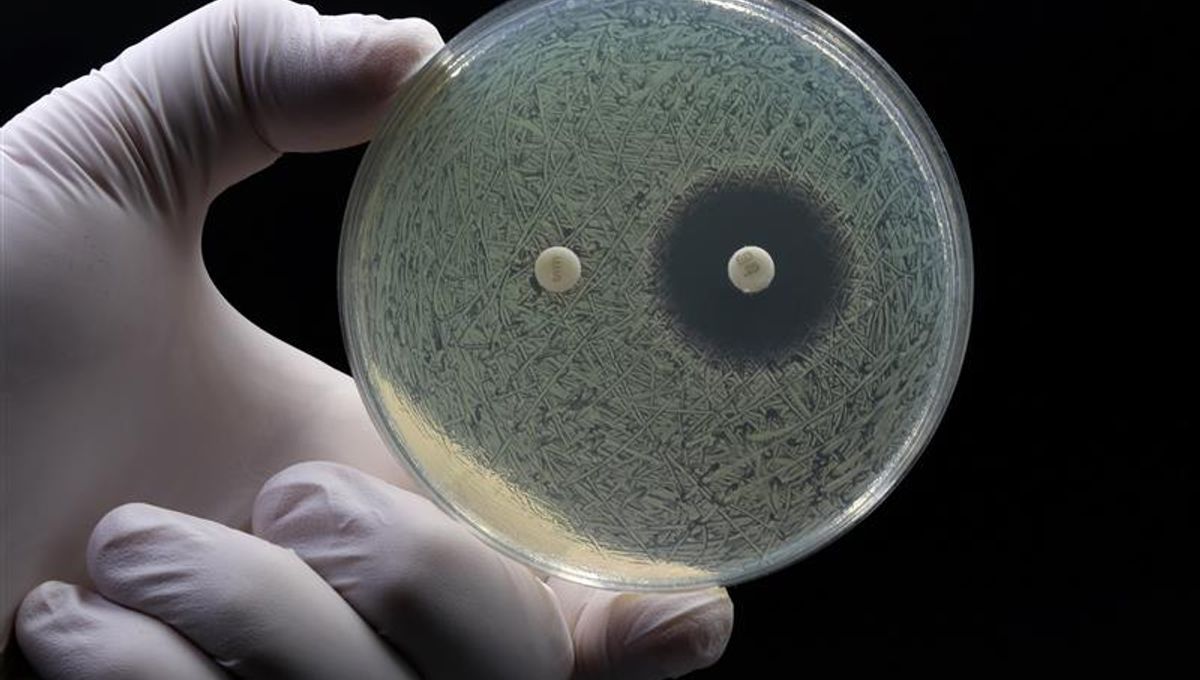
Antibiotics may represent the greatest achievement of modern medicine. Throughout the 20th century, this vital class of drug offered protection from previously lethal infections and transformed the therapeutic landscape.
However, the rise of antibiotic-resistant bacteria now threatens their supremacy and represents a critical healthcare challenge for the future. To understand how we can possibly fight back against emerging superbugs, we need to understand how antibiotics were first discovered and how their misuse has led to our current circumstances.
The dawn of antibiotics and the end of infections(?)
Humans have been treating infections with various plant extracts and molds for millennia. For instance, the Egyptian Ebers Papyrus from 1550 BCE, explains how to use moldy bread and soil as a remedy for wounds. Similarly, the use of moldy bread was also employed by other ancient peoples in China, Greece, and Serbia. But while these older techniques may have had benefits for some of their users, a healthy recovery was far from guaranteed. It was only in the first decade of the 20th century that things changed.
At the turn of the century, Dr Paul Ehrlich, developed the first modern antibiotic in the form of arsphenamine, or Salvarsan, a treatment for syphilis (he also coined the term “chemotherapy” too). Before this, syphilis was treated through the application of toxic metals, such as mercury and bismuth, so the discovery of arsphenamine’s effectiveness represented the elusive “magic bullet” Ehrlich had been seeking.
However, the use of this particular substance was unpleasant and was still often used in conjunction with other heavy metals. As such, researchers continued to experiment with different forms of it well into the 1930s when they discovered the active compound oxophenarsine (marketed as Mapharsen®), which became the standard treatment for syphilis until the late 1940s.
The most significant development in the history of antibiotics came at the end of the 1920s. It’s ironic that something so important was the result of an accident.
In 1928, Dr Alexander Fleming returned from his holiday and found that a Petri dish of Staphylococcus bacteria had become infected with mold as he had not sealed it correctly. However, disaster was averted when he noticed there were patches surrounding the mold where the bacteria could not grow. He quickly realized the mold created a chemical as a self-defense mechanism that killed bacteria. Fleming named this substance penicillin.
Fleming published his findings in 1929, but he was not able to purify the compound on his own. Over the next decade, Fleming and many others across Britain and America attempted to isolate penicillin for therapeutic use. Fleming was so keen to have the compound isolated that he even sent samples of the strain to anyone who requested it.
He was about to give up in the early 1940s, when a team of researchers at Oxford, led by Howard Florey and Ernst Chain, published a paper describing a process for purifying and concentrating penicillin. Although this early process produced minuscule amounts of the precious compound, it was nevertheless a start. By 1945, things had changed; penicillin was being mass-produced and distributed across the world, especially to soldiers fighting in the Second World War.
These developments brought with them the golden age of antibiotics which, between the late 1940s and 1960, witnessed the discovery of more drugs that joined penicillin in its fight against infections. Within this short time, around half of the drugs we commonly use today were discovered and commercialized. They include Streptomycin, which was originally discovered in 1943, as well as Tetracycline and Erythromycin, to name but a few.
At this time, faith in the power of antibiotics was so strong that one prominent doctor allegedly declared the end of infectious disease. Although such claims are apocryphal, they nevertheless highlight an enthusiasm and optimism for the power of antibiotics to fight disease. Alas, they were wrong.
The end of the Golden Age
Antibiotics’ success in beating infections was ultimately the key to their downfall. The increased use of these drugs for humans as well as nontherapeutic uses for animals (as a growth promoter) eventually led to the rise of antibacterial-resistant pathogens. Unfortunately, the battle against these tough microorganisms is ongoing – and we are not necessarily winning.
Bacteria are extremely numerous and experience a fast rate of naturally occurring random mutations that can give them genetic adaptations. Through basic mutation and natural selection, they can develop defenses to substances that would normally kill them. However, with the over- and misuse of antibiotics, mutations and adaptations among these microorganisms have accelerated. There are a number of mechanisms they use to achieve this.
When bacteria are exposed to antibiotics, the weaker organisms die off, which leaves the survivors to pass on any resistance to subsequent generations. But bacteria can also swap parts of their genetic material (known as genetic transduction) among other bacteria. This is a major driver in microbial evolution, but it is also a means for one type of bacteria to pass resistant traits to others.
This is especially concerning when you consider that bacteria can rapidly reproduce. In some instances, they can double in number in as little as 20 minutes.
In addition to bacteria’s ability to gain resistance to antibiotics, is the issue of developing new and novel treatments. Nearly all of the existing antibiotics we have at our disposal were developed during the original golden age. As such, our toolkit is old and increasingly out of date and there are no new alternatives being developed. This is why drug-resistant strains are so serious.
How can we fight back?
In order to deal with this threat, scientists are exploring a range of new treatment options. One example is the use of quorum-quenching, a technique that disrupts the ability of bacteria to communicate (a process known as quorum sensing). By interrupting bacteria’s ability to coordinate group behaviors and virulence factor production, the microorganisms are rendered less harmful and more susceptible to traditional antibiotics.
Another approach includes using bacteriophages (or phages), viruses that infect bacteria, to combat resistant strains in what is known as phage therapy. This approach has been around for nearly a century, but it is now being taken more seriously.
Then there is immunotherapy, which has already revolutionized cancer treatments. In this approach, white blood cells are removed or reprogrammed to combat cancer, which fights the disease without suppressing the patient’s immune system, like chemotherapy does. If applied to the treatment of infections, this same technique can be used to slow down the development of antibiotic resistance by only targeting the site of infection for its duration. This gives the microbes less time to develop resistance.
These are just a few examples of the potential treatment options that may arrive in the future, but they should not overshadow the work that can be done now and by all of us.
To be clear, the fight against antibiotic-resistant infections is not limited to scientists in laboratories. We all have a role to play in how we approach illnesses and the World Health Organization (WHO) has created resources to explain how.
Among the ways we can help fight back is the need to avoid unnecessary use of antibiotics. Contrary to what is sometimes believed, these powerful drugs are useless against colds and other viral infections. If you are prescribed antibiotics for a virus then you are providing bacteria with a chance to develop resistance. As such, you should neither seek antibiotics for these illnesses nor pressure your healthcare providers into giving them to you.
If you are prescribed antibiotics, then it is important for you to follow your health worker’s advice and ensure you finish the full course of treatment. Abandoning medication when you feel better may mean you still have bacteria in your system that can develop resistance and then multiply again.
Avoid taking someone else’s prescription or finishing off their spares for the same reason.
Finally, prevention is the best form of treatment. Make sure you regularly wash your hands, especially when preparing food, and avoid contact with sick people. Practicing safe sex is also important, as sexually transmitted infections are among some of the more resistant strains of bacteria.
All “explainer” articles are confirmed by fact checkers to be correct at time of publishing. Text, images, and links may be edited, removed, or added to at a later date to keep information current.
Source Link: Antibiotic Resistance Is A Reality – Here's How To Stop It