Some bacteria are capable of producing electricity out of tiny atmospheric concentrations of hydrogen. Having identified the enzyme responsible, scientists have demonstrated it can do this without the rest of the organism, opening the path to long-lasting ways to charge devices that can run on small amounts of electricity. The discovery could also allow for more efficient capture of energy stored as hydrogen and might even lead to a new way of producing bulk power in certain suitable locations.
For anyone who doubts the value of curiosity-driven research, a new example has just arrived that could become a classic. A team at Monash University, intrigued by the staying power of certain bacteria, have stumbled on what might be a path to clean non-polluting electricity, or at least better energy storage.
The authors discovered the key to the survival of the marvelously named Mycobacterium smegmatis is the Huc enzyme (pronounced Huck), which can trap hydrogen down to sub-atmospheric concentrations and use it as an energy source. Moreover, they announce in their new paper, they have learned Huc’s structure and can place a sample of it in an electric circuit and make current flow.
Professor Chris Greening told IFLScience that for his PhD he studied mycobacteria which, he said; “Are notorious for their ability to survive with nothing to eat. I made this very simple but elegant discovery that they switched to using energy from the atmosphere.” The secret was the air’s 0.5 parts per million of hydrogen.
Greening explained this comes from a variety of sources, including chemical reactions that break down methane and human activity such as the burning of fossil fuels. “About 20 percent is biological,” Greening added. “Some hydrogen is released during nitrogen fixation. Most gets recycled, but a significant amount escapes.” About three-quarters of this hydrogen is eventually removed by bacteria using Huc or something like it.
Burning hydrogen releases heat that can drive turbines, and fuel cells make electricity from hydrogen’s chemical potential – but both of these approaches rely on concentrations much higher than those that are normal in the atmosphere.
Ten years later, technology had improved sufficiently that Greening was able to return to the topic with colleagues and learn how the mycobacteria were performing this feat.
“What we really wanted to do was isolate Huc from a bacterium able to scavenge atmospheric hydrogen,” said Dr Rhys Grinter in a statement. “That is a challenging thing to do, because often these environmental bacteria are hard to cultivate. So, we developed a series of new methods for, first, growing the bacteria, then breaking them open and then using chemistry to try and isolate this single component.”
The team learned Huc is astonishingly resilient, surviving freezing or being heated to 80°C (176°F). As Grinter notes, it needs to be to help bacteria survive in the toughest environments.
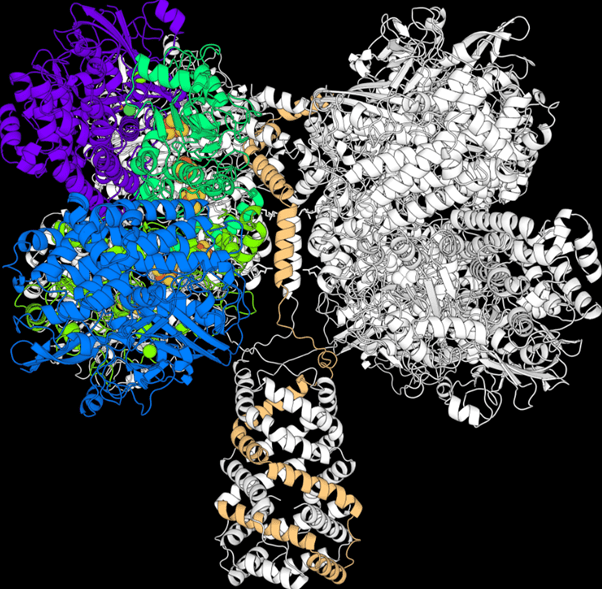
A molecular model of Huc. Image Credit: Rhys Grinter.
Huc’s most likely human application is to power instruments in remote locations that need only a small trickle of electricity, such as tags on animals. Other uses don’t involve electricity production.
“The synthesis of fine chemicals requires very specific modifications to a molecule, which can be difficult to perform chemically,” Grinter notes. “Huc could use the electrons from small amounts of hydrogen in air to perform these chemical modifications, in industrial chemical synthesis.”
With big plans to use hydrogen as a storage mechanism for solar and wind power, most of the focus has been on finding more efficient ways to make it by electrolyzing water. However, there are big losses when the energy is released, and improvements there would be equally valuable.
Greening told IFLScience that Huc would be slower than existing methods, and therefore unlikely to replace them. “Ideally you want a hybrid solution,” he said. “Use a traditional method to get most of the hydrogen, and then use Huc to get out the last gasp. It would be much more resilient.”
Greening added that existing fuel cells are easily damaged by impurities such as oxygen or carbon monoxide in the hydrogen. “Huc is incredibly tolerant of poisons,” he said, “And could be used to capture hydrogen from impure waste streams.”
There are places where surprisingly large quantities of hydrogen are trapped underground. A village in Mali has even been electrified without carbon emissions by tapping a subterranean supply. Hydrogen probably leaks in such places, creating higher ambient concentrations, and Greening said Huc might be able to capture it to provide clean power in substantial quantities when alternatives can’t.
How common such locations are is still unknown, but recent discoveries suggest there may be more than we expect.
The paper is published in the journal Nature
Source Link: Bacterial Enzyme Makes Electricity Out Of Thin Air