Exercise has undeniable benefits for body and mind, but a new study from engineers at MIT has found that the gains could reach far deeper. The team’s results show how physical activity that contracts the muscles leads to the release of biological signalers that could have the power to repair damaged nerve cells.
Uncovering the “exercise factor”
Humans realized pretty early on that exercising was good for us, but for many years it was unclear to scientists exactly what was going on when we flexed our muscles. Some kind of chemical messenger must be released from the muscle fibers, people reasoned, but without a firm idea of what it was, it was referred to as a somewhat nebulous “work factor” or “exercise factor”.
Then, in 2000, it was discovered that skeletal muscles release interleukin-6 (IL-6), a well-known cytokine that is most often associated with the immune system. Further work suggested that IL-6 alone was unlikely to account for all the effects of exercise, so the term “myokines” was coined to cover any and all similar factors that might be subsequently found.
Now, it’s clear that muscles have the capability of releasing hundreds of different peptides, but we’re still a long way from understanding all of their biological functions.
For example, while we know that getting a good workout in can benefit the nervous system by boosting our mood and helping us to de-stress, less is known about the impact of exercise at the level of individual nerve cells. This is what the team from MIT set out to explore.
Muscles “talking” to nerves
The spark of an idea came from a 2023 study by senior author Ritu Raman and colleagues, in which they found that muscle damage in a mouse could be healed by grafting in new muscle tissue and repeatedly stimulating it – essentially, exercising it. They hypothesized that the new muscle tissue was producing myokines that were promoting nerve and blood cell repair.
“That was interesting because we always think that nerves control muscle, but we don’t think of muscles talking back to nerves,” said Raman in a statement. “So, we started to think stimulating muscle was encouraging nerve growth.”
Showing this experimentally, however, was going to be a different matter.
“People replied that maybe that’s the case,” added Raman, “but there’s hundreds of other cell types in an animal, and it’s really hard to prove that the nerve is growing more because of the muscle, rather than the immune system or something else playing a role.”
This time around, the team developed a way of growing mouse muscle cells into fibers that were then fused together into stable sheets of muscle tissue, about the size of a quarter. Genetic modification meant that the muscles would contract in response to light, so they could be repeatedly “exercised” to mimic a workout.
After contracting the muscle, the team took samples of the fluid surrounding the sheets, to see whether the cells were releasing myokines, as Raman appetizingly describes: “I would think of myokines as a biochemical soup of things that muscles secrete, some of which could be good for nerves and others that might have nothing to do with nerves. Muscles are pretty much always secreting myokines, but when you exercise them, they make more.”
This soup was then applied to motor neurons that had been cultured in a similar way. With the addition of the myokines, the team saw that the neurons started to grow four times faster than usual – “and the effect is pretty immediate,” said Raman.
Genetic analysis revealed that the addition of myokines not only upregulated genes responsible for growth, but also boosted the functioning and maturation of the neurons.
Let’s get physical
Clearly, exercise is not merely a chemical process – there’s also the mechanical force of contracting and relaxing the muscles. You might never have thought about it, but each time you stretch a muscle, you’re also stretching all the attached nerve fibers.
To simulate this, Raman and the team grew another batch of motor neurons, this time on a surface peppered with tiny magnets. They exercised the muscles for 30 minutes every day, applying an external magnet to wiggle the surface backwards and forwards.
Just as they saw with the direct application of myokines, physically exercising the neurons made them grow longer.
“That’s a good sign because it tells us both biochemical and physical effects of exercise are equally important,” Raman said.
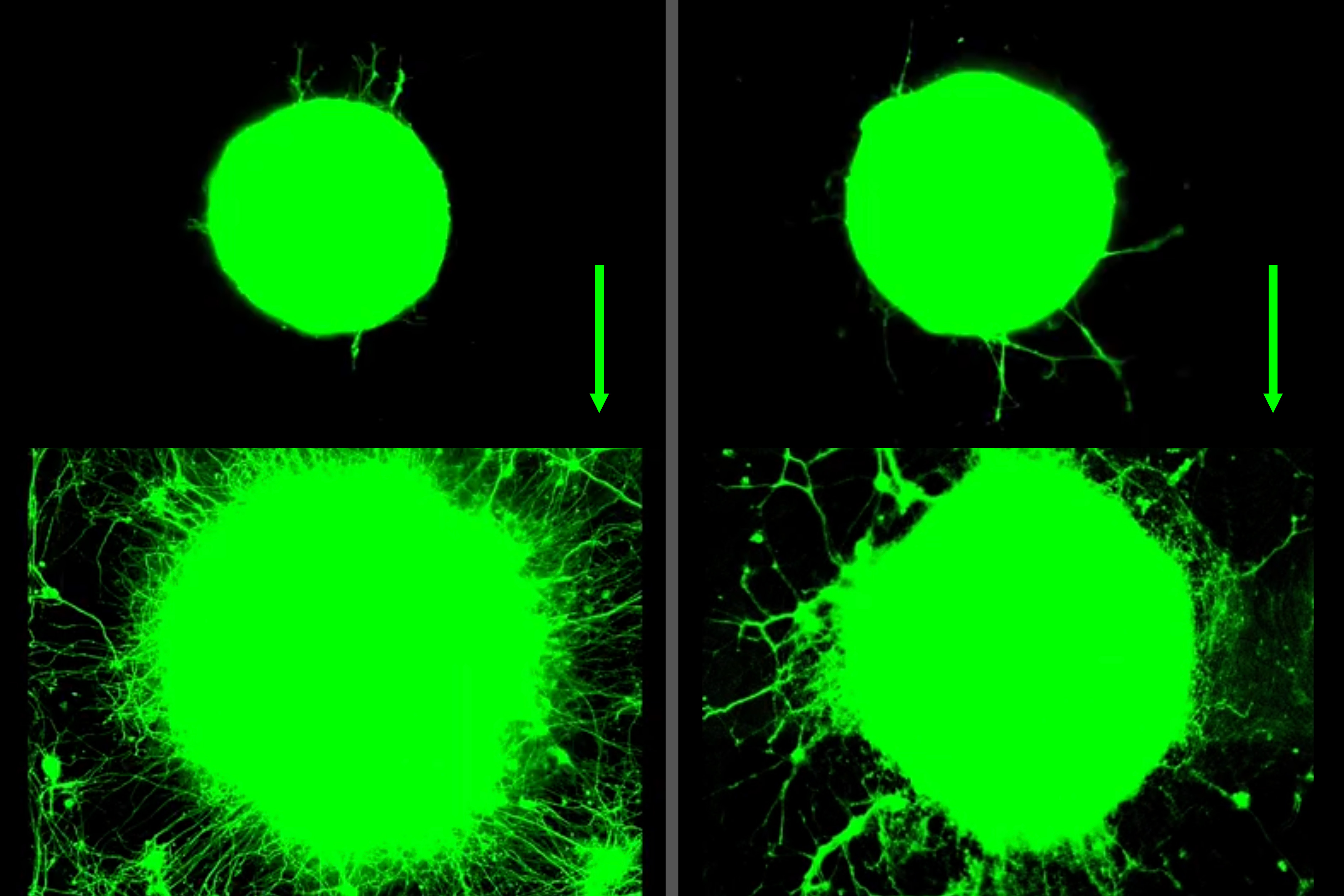
These green orbs are bundles of nerve cells that grow outwards by sending out long tails called axons. The images show the growth over the course of five days in response to biochemical (left) and physical (right) exercise signals.
Myokines are a bit of a hot topic right now. Another recent but unrelated study showed how a medicine containing myokines could one day be used as “exercise-in-a-pill” to help protect the brain from age-associated dementia.
With their new research, Raman and colleagues hope to unlock new ways of restoring damaged nerves, which could help people with other neurodegenerative diseases such as amyotrophic lateral sclerosis. There’s much more work to be done, and lots more still to discover about these mysterious messengers.
As Raman said, “This is just our first step toward understanding and controlling exercise as medicine.”
The study is published in the journal Advanced Healthcare Materials.
Source Link: Can Exercise Help Heal Damaged Neurons?