The US Food and Drug Administration (FDA) has announced new draft recommendations to improve the accuracy of pulse oximeters – devices used to estimate blood oxygen levels – across the whole range of skin tones, after a wealth of studies identified inaccuracies in readings taken for people with darker skin.
Pulse oximeters: what they are and how they work
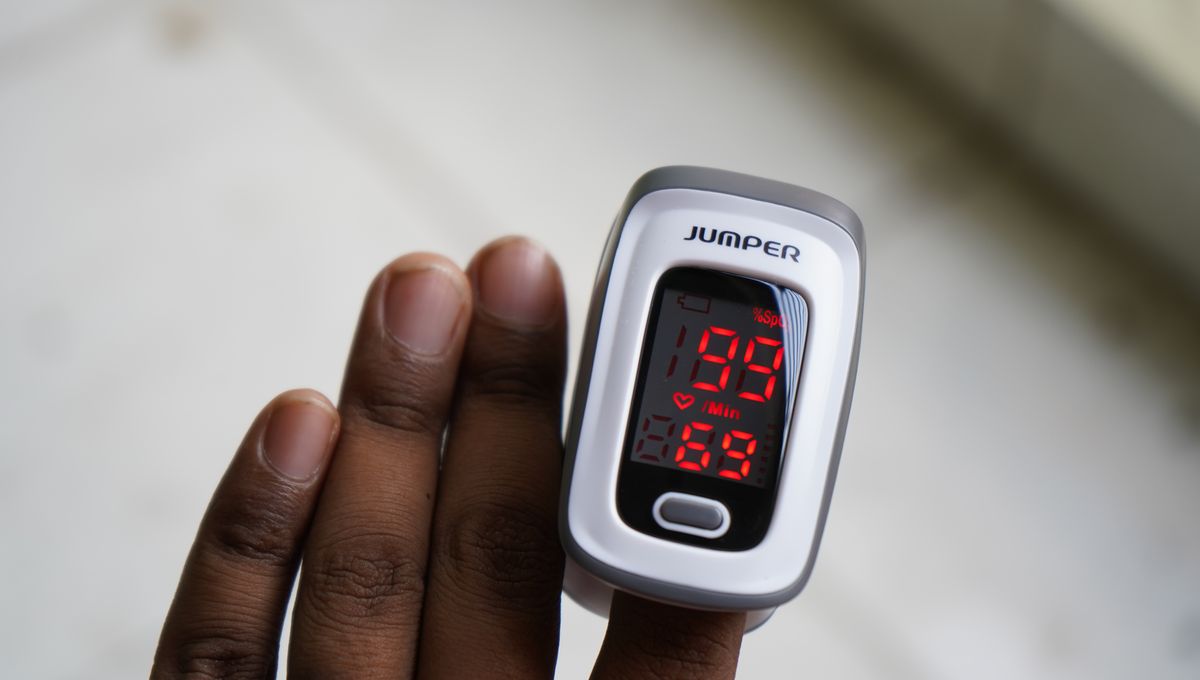
Pulse oximeters are small devices that are designed to estimate a person’s pulse rate and the level of oxygen in their blood, the latter to give an idea of how well someone’s lungs are working.
They do so by having one side that emits light that passes through the finger. On the other side is a sensor, which detects and measures the amount of light that didn’t get absorbed by the tissue and blood in the finger. This measurement is used to provide the estimate of the level of oxygen being carried by red blood cells, known as oxygen saturation.
What prompted the new plan – and why does it matter?
However, there’s a problem with the way that pulse oximeters work: they’re affected by the presence of pigments in the skin.
Darker skin tones are typically the result of the presence of more of the pigment melanin, which can interfere with the absorption of light from the pulse oximeter. In practical terms, this means that an oxygen saturation reading for someone with darker skin may read as much higher than it actually is.
Researchers have been aware of the problem for decades; a study published in 1990 was amongst the first to investigate, identifying that inaccurate readings were more common for Black ventilator-dependent patients receiving oxygen therapy than they were for white patients in the same circumstances (27 percent vs 11 percent).
However, the COVID-19 pandemic brought fresh attention to the issue. In 2023, a study published in JAMA Network Open found that in a group of over 24,000 patients hospitalized with COVID-19 in the US, “pulse oximeters more commonly overestimated arterial oxygen saturation in patients from minority racial and ethnic groups.”
The fact that pulse oximeters have inaccuracies is a significant problem in and of itself given how widespread their use is, but it’s the potential consequences of inaccurate estimates and what they mean for how patients with darker skin tones are treated that are also cause for concern.
“It’s important to keep in mind that pulse oximeters give us an estimate, but it’s more than just a number. We use that estimate to make clinical decisions, such as how much supplemental oxygen to give a patient,” said Eric Gottlieb, HMS clinical fellow in medicine at Brigham and Women’s Hospital, in a statement made in 2022. “It has real meaning for the patients that we care for because we can track back racial disparities in treatment to these differences in measurements.”
And that’s exactly what a study authored by Gottlieb and colleagues showed, finding that were “differences in supplemental oxygen administration between Asian, Black, and Hispanic patients and White patients that were associated with pulse oximeter performance.”
A similar finding was made in the 2023 JAMA Network Open study, concluding that the inaccuracies in pulse oximetry readings “led to delayed recognition of need for COVID-19 therapy among Black patients compared with White patients.”
What does the draft guidance say?
In 2021, following the publication of another large study illustrating the differences in accuracy, the FDA issued a safety communication acknowledging the problem in light of the pandemic – though this was primarily directed at patients and healthcare providers.
Now, the new draft guidance is targeted at manufacturers of pulse oximeters in order to “help ensure the public has access to pulse oximeters that perform accurately across the range of skin pigmentations as soon as possible,” the FDA said in a statement.
The draft recommendations suggest that in gathering the clinical data used to develop pulse oximeters, manufacturers should not only be testing the devices on more people – it recommends 150 or more – but also evaluate their accuracy across “a diversely pigmented group”.
On that point, they recommend using both objective measures and a subjective standardized assessment of pigmentation known as the Monk Skin Tone scale, and then ensuring that at least 25 percent of participants in data-gathering studies fall within each of the three groups of this scale, ranging from lighter to darker skin tones.
“The FDA believes the implementation of these recommendations, if finalized, will help improve the accuracy and performance of pulse oximeters used for medical purposes in patients across the range of skin pigmentation,” said the agency.
Will it make a difference?
The draft guidance will now be open to public input for 60 days. Even after that time, and if the recommendations go ahead as outlined, they are just that – recommendations. Manufacturers don’t have to follow them, and that makes it difficult to predict whether it’ll make any real difference to solving the pulse oximeter problem.
Michael Sjoding, an associate professor of internal medicine at the University of Michigan, takes a positive view. “Part of their marketing plan is to say, like, ‘Look, our pulse oximeter performs accurately,’” Sjoding told the Washington Post.
Others aren’t so sure.
Theodore J. Iwashyna, who co-authored a study that illustrated low uptake the last time the pulse oximeter guidance was updated in mind of disparities, said to the Post, “Unless there is formalization, enforcement and compliance, I am not sure why we would anticipate these ‘proposed new draft recommendations’ would result in better products, and therefore better, more equitable care.”
The content of this article is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of qualified health providers with questions you may have regarding medical conditions.
Source Link: Decades-Old Problem Of Pulse Oximetry Across Skin Tones Targeted By New Draft FDA Guidance