Two diamonds from South Africa’s Voorspoed mine provide a rare direct insight into the chemistry of Earth’s mantle. Although diamonds can be formed in multiple ways, the findings add to evidence natural diamonds are most often formed deep in the mantle at depths where pressures are 9-16 billion pascals and temperatures are around 1,600°C (2,900°F).
The rest of this article is behind a paywall. Please sign in or subscribe to access the full content.
The Earth’s core is thought to be composed primarily of iron and nickel, some of which are mixed into the mantle. Efforts to model how these elements would behave under mantle conditions have concluded that nickel-rich metal alloys should become stable at depths of 250-300 kilometers (150-180 miles). However, there could be important factors missing from these models, since experiments to replicate the relevant pressures can only be done on tiny samples. Even if the nickel-rich layer exists, the models might not have its depth right.
Consequently, geologists have searched for evidence of nickel-rich minerals formed at these depths, but until now, without success. That has changed with the discovery of flecks within diamonds formed at 280-470 km (170-280 miles). Analysis reveals the nanoscale inclusions are composed of nickel-iron, while microscopic counterparts are nickel-rich carbonates, providing the first direct evidence of nickel abundance at these depths, and proving once again diamonds are a geologist’s best friend.
Not everything trapped in the diamonds contains nickel. Geochemists also found a form of silicon dioxide known as coesite, potassium, and nitrogen solidified by the pressure. These serve as indicators of the pressures, and therefore the depths, at which the diamonds formed, confirming the nickel layer is where models predicted.
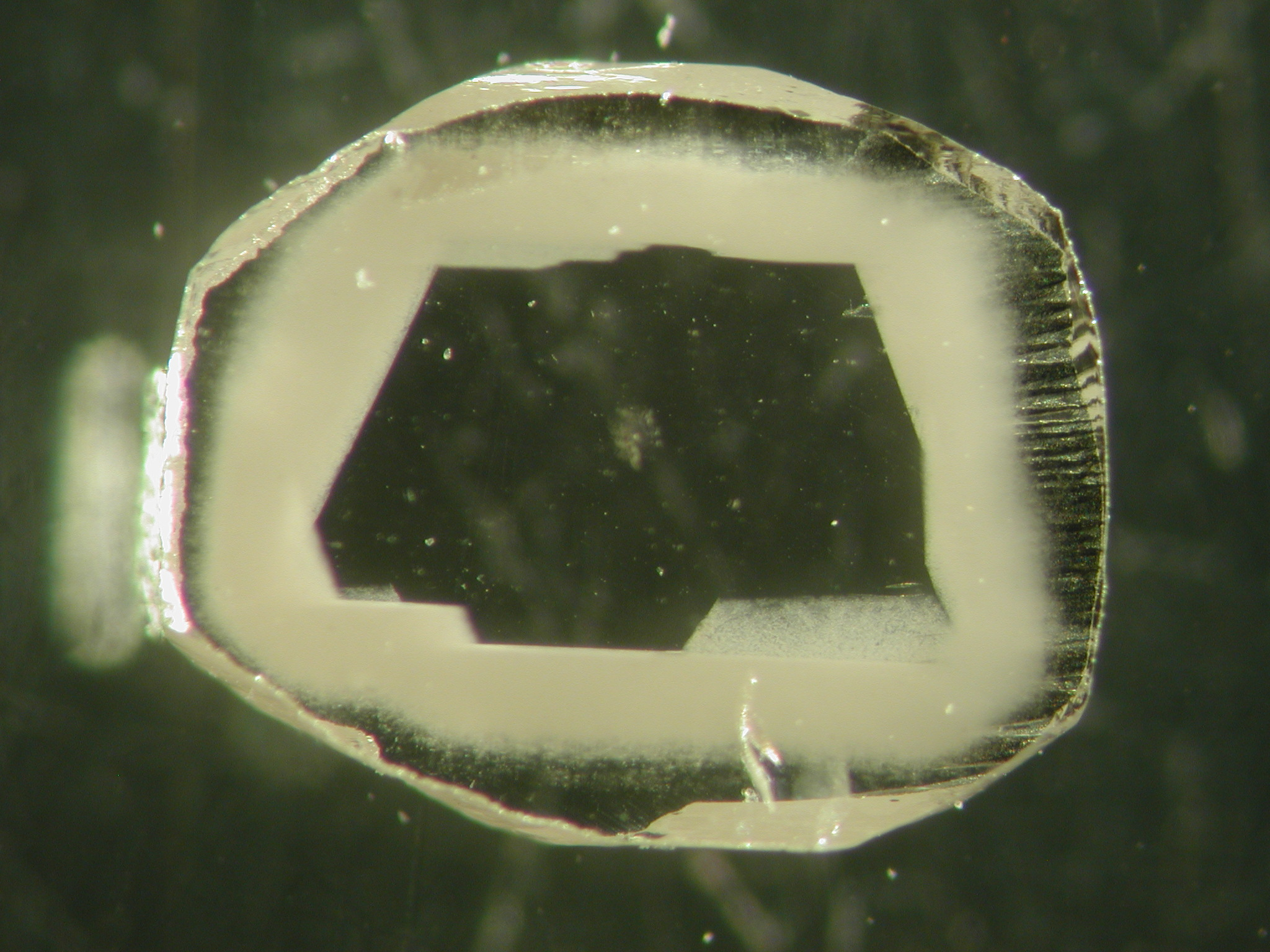
When sliced open, the diamond reveals a clear core, surrounded by the cloudy zone loaded with intrusions.
Image Credit: Yaakov Weiss
To get both nickel-iron and nickel-rich carbonates under these conditions, chemists think a process known as metasomatic redox-freezing must have taken place. This involves a melt of oxidized carbonate-rich material making its way into a metal-bearing rock called peridotite, which is rich in silicate minerals like olivine, and also contains most of the mantle’s nickel.
The interaction of these components oxidizes the metals, but iron responds more easily, leaving higher concentrations of nickel behind. The authors think the diamonds crystallized just as this was happening, capturing the nickel-enriched alloy and sealing it off from other processes. Diamonds may not be forever, but they go close, trapping the impurities for transport to the surface and eventual study.
“This is a rare snapshot of mantle chemistry in action,” Dr Yaakov Weiss of the Hebrew University of Jerusalem said in a statement. “The diamonds act as tiny time capsules, preserving a reaction that would otherwise vanish as minerals re-equilibrate with their surroundings.”
Deeper in the mantle, the increased pressure leaves the iron the dominant partner in nickel-iron alloys.

The dark dots on this diamond come from laser ablation pits used to take microscopic samples for chemical analysis.
Image Credit Yaakov Weiss
Besides confirming the expectation of nickel-rich alloys, Weiss and co-authors think the discoveries could also help explain a previous puzzling finding indicating some garnets formed under unexpectedly oxidizing conditions. Presumably, these were situations where the oxidized carbonate was more locally abundant and therefore oxidized the nickel after running out of iron.
The presence of carbonates and potassium in mantle peridotite has been another enigma to scientists, and one with important implications for the composition of volcanic magmas. The authors propose that potassium-rich carbonatitic melts penetrate peridotitic rocks just as the oxidized carbonatitic melt did when the Voorspoed diamonds were formed, and could explain the presence of elements that escape magma easily. Volcanic rocks formed from these enriched magmas include kimberlites, famous for hosting the world’s richest diamond supplies.
The study is published in Nature Geoscience.
Source Link: Diamond "Time Capsules" Capture Rare Snapshot Of Redox-Freezing Reaction