Element 116 has been made with a new method – a step towards synthesizing element 120, a hypothetical element hypothesized to be in the long-predicted “island of stability”, in the lab.
Elements are defined by the number of protons in their nucleus, with hydrogen having one, helium having two, and uranium has 92 protons. The number of neutrons can vary, with different elements needing different numbers of neutrons to be stable (or at least long-lasting), with many elements having several different versions, known as isotopes.
Though uranium is the heaviest naturally occurring element that we know of, scientists have synthesized heavier elements in the lab.
“The production of SuperHeavy Elements (SHE), and the investigation of their nuclear properties, stands as an important frontier in modern nuclear physics, pushing the boundaries of our understanding of the fundamental constituents of matter,” the Berkeley Lab team explains in their new paper.
Though not exactly in a linear fashion, elements become less stable the heavier they get. Element 115, moscovium, has a half-life of just 220 milliseconds in the form of moscovium-289, decaying before scientists can do much in the way of studying it. The heaviest element created by scientists – element 118, oganesson, first obtained in 2002 – has a half-life of less than a millisecond.
So why go heavier if even the most stable superheavy elements decay with lightning speed? Looking at the stable isotopes of elements we do know about, scientists have predicted the “island of stability” further up the periodic table containing elements that might not decay so quickly.
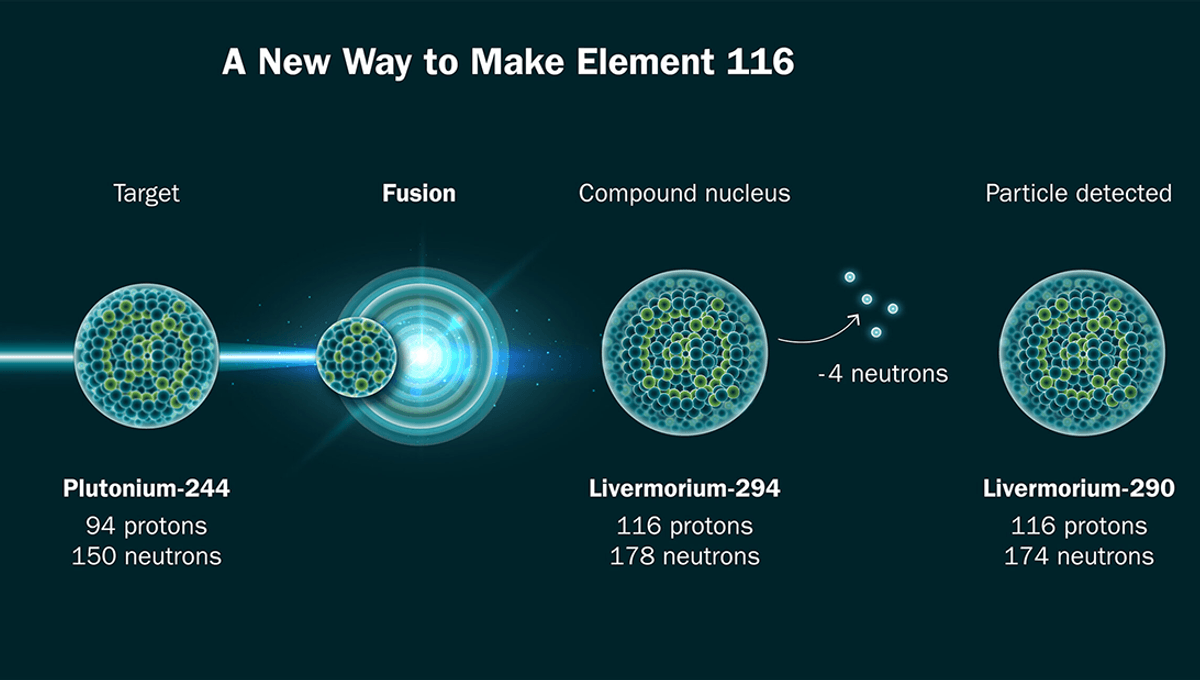
In the new study, as a stepping stone to creating element 120, the team attempted to make element 116 using a different method. Usually, heavier elements between 114 and 118 are made by bombarding target nuclei with a calcium-48 beam. The team instead used titanium-50, despite uncertainty about whether it could be used to make heavier elements.
Doing so was not easy, requiring the team to vaporize titanium-50 in a tiny oven before using a complex superconducting magnet and free electrons bombarded with microwaves to increase their energy in order to knock away 12 of the titanium’s 22 electrons. This was then maneuvered and accelerated using magnets, and used to bombard plutonium in order to make element 116. About about 6 trillion titanium ions hit the target every second before the element is separated from the debris using magnets.
“We’re very confident that we’re seeing element 116 and its daughter particles,” Jacklyn Gates, a nuclear scientist at Berkeley Lab said in a statement, referring to elements that 116 decays into. “There’s about a 1 in 1 trillion chance that it’s a statistical fluke.”
Now that element 116 has been synthesized using this groundbreaking method, the team has its sights set on creating element 120.
“It was an important first step to try to make something a little bit easier than a new element to see how going from a calcium beam to a titanium beam changes the rate at which we produce these elements,” Jennifer Pore, a scientist in Berkeley Lab’s Heavy Element Group, added. “When we’re trying to make these incredibly rare elements, we are standing at the absolute edge of human knowledge and understanding, and there is no guarantee that physics will work the way we expect. Creating element 116 with titanium validates that this method of production works and we can now plan our hunt for element 120.”
Element 120, as well as being further up the table than any element we have produced, is nearing the island of stability. Were we able to produce an element there, it could be stable enough for scientists to study its properties – or perhaps even find a use for it. The team will attempt to make element 120 by bombarding californium-249, after first synthesizing it and making adjustments to their equipment, hopefully beginning work in 2025. The team believes it may take around 10 times longer to produce, but are optimistic that it is feasible.
“We’ve shown that we have a facility capable of doing this project, and that the physics seems to make it feasible,” Kruecken said. “Once we get our target, shielding, and engineering controls in place, we will be ready to take on this challenging experiment.”
The study is posted to pre-print server arXiv and is submitted to the journal Physical Review Letters.
Source Link: Element 120 On The Horizon As New Way To Synthesize Element 116 Found