If we were to zoom right into some biological tissue, be it plant or animal, then we might just see some cells that look as though they’re sending out or receiving tiny bubbles. These are exosomes, and according to some, they’re the cure for pretty much everything – from hair and skin issues to problems with the immune and nervous systems. But how much evidence is there for those claims?
What are exosomes?
Exosomes are a small, specific type of extracellular vesicle (EV), a kind of membrane-enclosed package that can move between cells. What separates them from other EVs is that they can carry a wealth of different cellular bits and bobs, ranging from proteins and lipids to DNA and RNA.
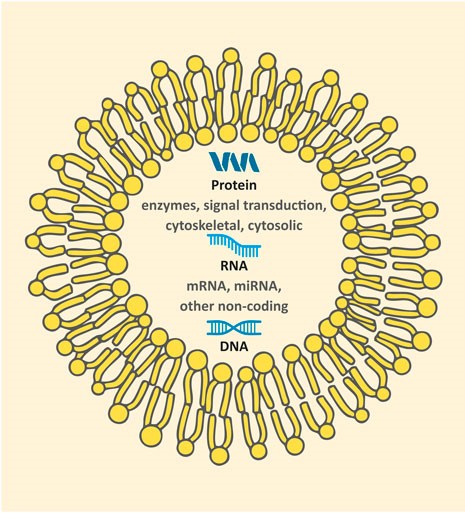
This is an exosome; the yellow blobs and wiggles represent a phospholipid membrane.
Their ability to carry all kinds of different materials has led to suggestions that exosomes play a role in removing cellular waste and communication between cells – particularly when it comes to the immune system and controlling cell death or survival.
Exosomes can come from different sources, both animal and plant: they can be derived from stem cells, sourced from human umbilical cords; bodily fluids like milk; and even fruits and seeds.
What exactly is exosome therapy?
With their small size, storage capabilities, and ability to pass through cellular barriers, exosomes have caught the attention of those searching for new therapeutics.
One area in particular where this seems to have taken off is in aesthetics, where exosome therapy can be presented with claims of being a “regenerative” treatment. Feel like your skin is looking a bit tired and old, or experiencing hair loss? You might be offered a treatment with exosomes derived from salmon testicle cells.
However, there have also been suggestions that exosomes have the potential to treat various diseases, ranging from transmissible illnesses like COVID-19 to degenerative conditions such as Parkinson’s disease and osteoarthritis.
Do exosome products have any benefits?
Let’s not beat around the bush: in the US, there are no FDA-approved exosome products, which gives us some indication as to their current legitimacy as a treatment.
Exosomes, when used to treat diseases or conditions, are regulated in the same way as drugs and other biologics. This means that they have to go through a rigorous, often years-long process to generate enough quality supporting evidence to demonstrate both their safety and efficacy. The lack of FDA approval indicates that the science to support exosome therapy simply isn’t strong enough at this point.
Nonetheless, businesses are still dishing out exosome treatments; a 2021 study found that there were 99 businesses in the US making therapeutic claims about the exosome products they offered. Another study identified 16 US businesses marketing exosome products specifically to treat COVID-19.
The FDA has issued warnings against making such claims. “None of these products have been approved for the treatment or prevention of COVID-19, acute respiratory distress syndrome (ARDS), or any other complication related to COVID-19,” the agency said in a statement made in 2020.
A separate statement had to be issued the previous year after it was discovered that people treated with unapproved exosome-containing stem cell products in clinics in Nebraska had experienced serious adverse events.
“The clinics currently offering these products outside of FDA’s review process are taking advantage of patients and flouting federal statutes and FDA regulations,” said the agency. “This ultimately puts at risk the very patients that these clinics claim to want to help, by either delaying treatment with legitimate and scientifically sound treatment options, or worse, posing harm to patients, as evidenced by these recent reports of adverse events.”
Is there any evidence at all?
While the necessary evidence to support exosome products becoming FDA-approved simply isn’t there right now, that’s not to say there haven’t been any studies investigating their potential.
For example, a 2021 preclinical study – these are designed to establish dosing and safety before testing a treatment on humans – carried out in rabbits set out to establish whether a gel containing exosomes derived from platelets could enhance ischemic wound healing and skin regeneration.
The study found that wounds treated with the exosome product not only healed, but did so with less scarring, with the normal properties of the skin and the blood vessels that supply it restored.
Whilst the findings are positive, this doesn’t mean people should start putting exosome products on their skin with reckless abandon; the effects seen in rabbits are far from guaranteed to be the same in humans. The study authors themselves only call their work a “proof of concept”.
There are also exosome products currently being studied for their therapeutic potential under the FDA’s Investigational New Drug (IND) program, which allows for them to be tested on humans in clinical trials.
In January 2024, an IND application by biotech company Aruna Bio for an exosome product was cleared by the FDA. The company says that the product, dubbed AB126, can cross the blood-brain barrier, and has the potential to treat patients who have experienced acute ischemic stroke. Clinical trials to study this possibility were set to start this year – the first time an exosome product has entered clinical trials for a neurological condition.
Nonetheless, a recent review of research into the clinical applications of exosome therapy found that there’s still a long way to go before there’s a chance of such products being approved. The authors acknowledge that exosomes show promise as diagnostic biomarkers; in promoting tissue repair; and as therapeutic agents in diseases like cancer, autoimmune disorders, and neurological conditions.
Nonetheless, they found that it’s proven difficult for scientists to ensure that exosome products are pure and stable when produced on a bigger scale – factors that are key to a therapeutic’s safety and efficacy, and thus the chance of it getting approved.
There’s also a wider problem of how exosomes are sourced and how that might affect their action. “Because almost all cells create and release exosomes, the source of these small vesicles is critical in defining their function,” wrote pain specialist Aaron Hanyu-Deutmeyer, DO; and director of the Duke Regenerative Pain Therapies Program, Thomas Buchheit, MD, in a 2023 edition of ASRA Pain Medicine News. “This means that exosomes have the potential to harm as well as heal.”
The authors of the review further concluded that there still needs to be more research into the biological mechanisms that underpin exosome therapies, which can also help to determine whether or not they are safe.
All “explainer” articles are confirmed by fact checkers to be correct at time of publishing. Text, images, and links may be edited, removed, or added to at a later date to keep information current.
The content of this article is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of qualified health providers with questions you may have regarding medical conditions.
Source Link: Exosomes: Are They Really The Cure-All Some Claim Them To Be?