A molecule with two carbon atoms sharing a single electron, in defiance of chemistry textbooks, has been revealed. Examples of atoms from differing elements sharing single electron covalent bonds have been reported recently, but this is the first case of it occurring between two carbon atoms. Given the central status that carbon bonds have in the formation of life, a new way for them to come together has an importance far beyond that of most bonds between atoms.
Covalent bonds typically involve pairs of electrons being shared between two atoms, binding them together. Sometimes electrons will form multiple covalent bonds, making something particularly hard to break. The truth is of course more complex – isn’t it always? – but for more than a century, the idea that electron pairs were required has largely held.
Single electron bonds have been found between other atoms, for example when a phosphorus molecule loses one of its electrons it doesn’t always fall apart. However, such bonds are usually weak. The discovery of one between two carbon atoms strong enough for a large molecule to stay together will allow chemists to explore the grey area between bonded and non-bonded states.
Since any single electron bond between carbon atoms is bound to be weak, chemists searching for an example needed to find a way to stabilize molecules, rather than have other reactions destroy them. At the slightest opportunity the atoms will either lose the bond entirely, or grab a passing electron to form a traditional covalent pair.
The researchers focused on hexaphenylethane (HPE) derivatives, which they say form relatively stable carbocations and radicals (an atom or molecule with an unpaired electron). HPEs have a stretched bond between two carbon atoms. Their product has a shell of carbon rings surrounding a carbon-carbon bond, which becomes stretched until it loses one of its electrons. By treating both sides of the bond with iodine of different concentrations the team produced; “Dark violet single crystals suitable for X-ray diffraction measurements.”
The authors claim the distinctive geometry of the atoms within the crystal prove a single atom bond, subsequently confirmed with Raman spectroscopy.
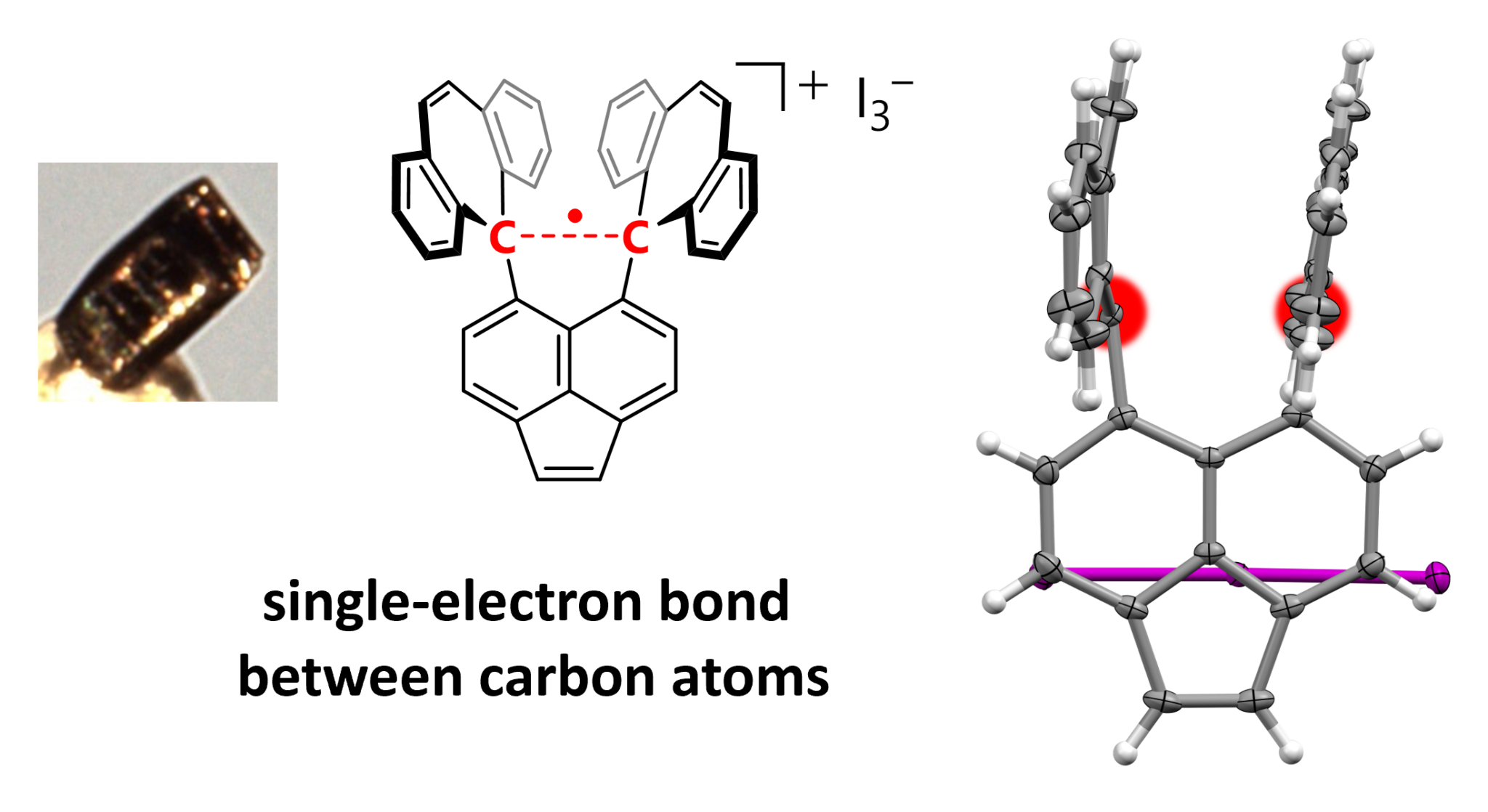
Structure of the compound highlighting the C–C sigma bond (red).
Image Credit: Takuya Shimajiri, et al. Nature. September 25, 2024
“The covalent bond is one of the most important concepts in chemistry, and discovery of new types of chemical bonds holds great promise for expanding vast areas of chemical space,” study co-author Dr Takuya Shimajiri of the University of Tokyo told Nature News.
Professor Guy Betrand of the University of California, Santa Barbara (who is not an author of this study) was part of a team that demonstrated a single electron bond between phosphorus atoms. In speaking to Nature News he gave credit to those involved in the new discovery, saying: “Anytime you do something with carbon, the impact is greater than with any other element.”
The possibility of a single electron bond between two carbon atoms was proposed by Linus Pauling in 1931. Pauling is honored as one of the few scientists to win two Nobel Prizes, but also postulated an incorrect model of DNA and was subsequently mocked for his promotion of immense doses of Vitamin C.
No applications have yet been proposed, but co-author Professor Yusuke Ishigaki of Haikkaido University said; “Elucidating the nature of single-electron sigma-bonds between two carbon atoms is essential to gain a deeper understanding of chemical-bonding theories and would provide further insights into chemical reactions,” in a statement.
The paper is published in the journal Nature.
Source Link: First Example Of Single Electron Carbon-Carbon Pairs Could Rewrite Textbooks