A derivative of the metal-organic complex ferrocene has 20 valence electrons in a stable configuration, overturning the expectation for the last 100 years of a ceiling at 18 valence electrons.
Combinations of metals and carbon-based molecules show rich possibilities for unusual chemistry. One class of these metal-organic complexes is metallocenes, where organic rings sit either side of a metal atom. Ferrocene (Fe(C5H5)2), the first metallocene discovered, was considered such a breakthrough when it was made in 1951 that it won its makers the 1973 Nobel Prize. Its creation is considered to mark the start of modern organometallic chemistry, but it seems there is still plenty for us to learn about it.
Ferrocene normally has 18 valence electrons (those that can contribute to forming chemical bonds), and according to Dr Satoshi Takebayashi of Okinawa Institute of Science and Technology Graduate University, that’s part of a pattern first observed 30 years earlier. “For many transition metal complexes, they are most stable when surrounded by 18 formal valence electrons. This is a chemical rule of thumb on which many key discoveries in catalysis and materials science are based,” Takebayashi said in a statement.
Indeed, the expectation that certain molecules will have 18 valence electrons is so useful for chemists looking to predict molecular structure, it has contributed to three more recent Nobel Prizes.
However, just because 18 electrons represent a sweet spot does not mean there are no alternatives. Some 16-electron complexes are stable, and paramagnetic nickelocene has 20 valence electrons. Now the list of exceptions to the rule has expanded following Takebayashi and co-authors’ creation of a 20-electron ferrocene derivative using an iron-nitrogen bond.
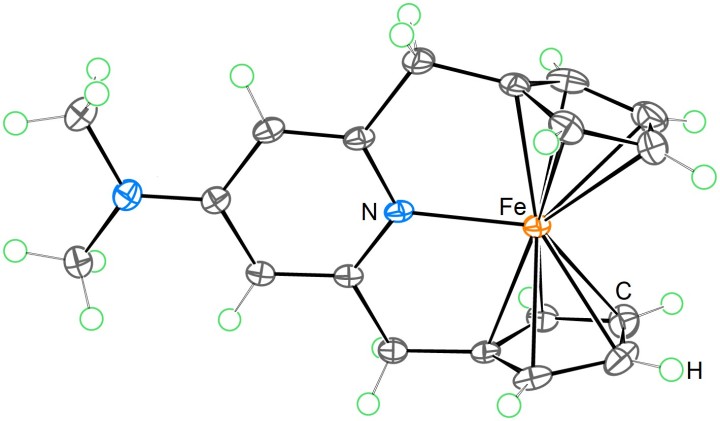
The new 20-electron ferrocene derivative’s structure, with nitrogen (blue), iron (orange), hydrogen (green), and carbon (grey) atoms highlighted.
“The additional two valence electrons induced an unconventional redox property that holds potential for future applications,” Takebayashi said. Ferrocene and its derivatives are used in reactions where electrons are transferred (redox), both as a catalyst and reactant, with applications as diverse as solar cells and medical instruments. However, its previous narrow range of oxidation states has limited the reactions it can drive. The discovery should change that. Indeed, it is noted for its stability in many environments.
A 19-electron anion ferrocene derivative has been made previously, but only with a powerful reductant; 20-electron ferrocenes turned out to require a less extreme approach. When a nitrogen atom bonds to the iron atom at ferrocene’s heart, two electrons are directed away from the iron, ready to bond to other molecules.
The study is open access in Nature Communications.
Source Link: For 100 Years, A Stable 20-Electron Ferrocene Molecule Was Thought "Improbable" – Until Now