Building blocks for life have been found in meteorites, apparently having formed without the benefits of the complex chemistry planets can support. The Earth may have been seeded with the necessary ingredients this way. Evidence of some of these complex organic molecules has been found in the vast gaps between the stars. Now lab-based studies offer an explanation of how they could form there.
Even before life existed, Earth had many features that could have assisted the formation of complex organisms. The Sun and hydrothermal vents provided energy. Water could serve as a solution, and rain and wind moved things around. It’s much harder to work out how carbon-based molecules could form in interstellar space, but we know from spectra that many of them do.
Japanese researchers have mimicked conditions in interstellar clouds in the lab, revealing the vital role the surfaces of ice grains play.
Carbon is just one element among almost 100 that exist naturally – yet the overwhelming majority of known molecules not only include it, but are based around bonded chains of carbon atoms. Carbon’s chemistry allows the build-up of much larger molecules than any other element, so it is just as well so much of it is formed when stars stop fusing hydrogen and move on to helium.
Yet just because carbon can form complex molecules does not mean it automatically will. In the vastness of star-forming clouds, atoms are so dispersed they seldom come in contact with each other. Finding a compatible partner is harder than at a really bad nightclub.
It’s thought that ice grains may serve as a sort of atomic hook-up app, bringing carbon molecules together, but for this to work they would need to be able to diffuse across the grains’ surface. No one has been sure under what circumstances this might be possible.
Professor Masashi Tsuge of Hokkaido University and colleagues have tried to replicate the conditions in gas clouds such as the Orion molecular cloud complex. This involved cooling the materials known to exist in these clouds to temperatures not far above absolute zero.
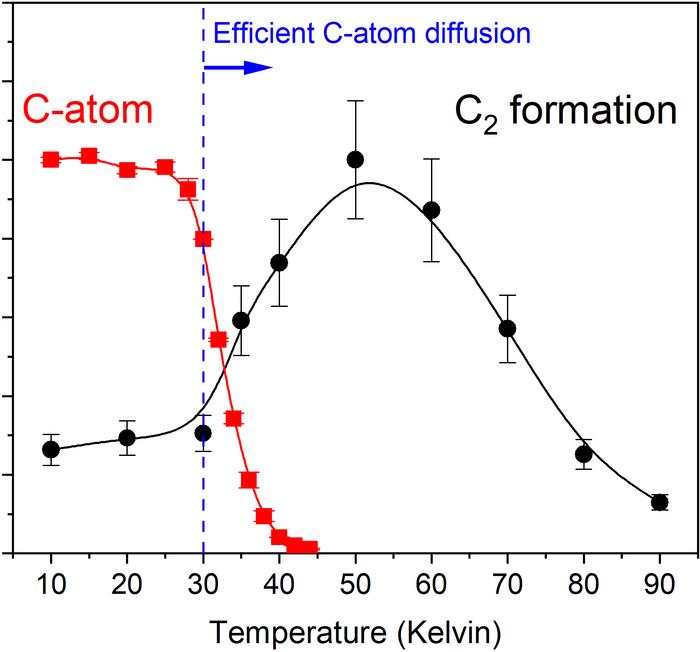
“In our studies, recreating feasible interstellar conditions in the laboratory, we were able to detect weakly-bound carbon atoms diffusing on the surface of ice grains to react and produce C2 molecules,” Tsuge said in a statement.
Tsuge and colleagues found that diffusion can occur at temperatures above 30 Kelvin (-405 °F, or -243 °C). The activation energy required to allow carbon atoms to diffuse across ice is low enough that theoretically, even 22 Kelvin (-420 °F, or -251 °C) should be sufficient.
The temperatures at which carbon molecules diffuse on the surface of ice grains, and bond to form C2 molecules
Image Credit: Masashi Tsuge, et al. Nature Astronomy. September 14, 2023
Even this is warmer than regions of space far from any star. However, Tsuge notes that large areas of the protoplanetary disks around very young stars reach these temperatures. Even though very young stars have yet to reach peak brightness, they can still provide a little warmth, and it seems a little is all carbon needs.
It might take 100,000 to 10 million years for two carbon atoms to make the glacially slow migration across a hundred nanometers to meet, but these atoms have plenty of time.
Once the carbon bond is formed, more atoms can get added by the same diffusion process, building up steadily more extensive carbon skeletons, to which other atoms can attach.
Most carbon atoms within a cloud will not follow this path, the team notes. Instead, they will encounter hydrogen or oxygen atoms and form methane or carbon monoxide, limiting the prospects for future growth. Even those who land on the ice grain surface unattached will often form formaldehyde (CH2O) instead.
However, enough combine to form long carbon chains for us to detect the outcomes, and perhaps to be here in the first place.
The study is published in the journal Nature Astronomy
Source Link: How Complex Organic Molecules Form In Interstellar Space