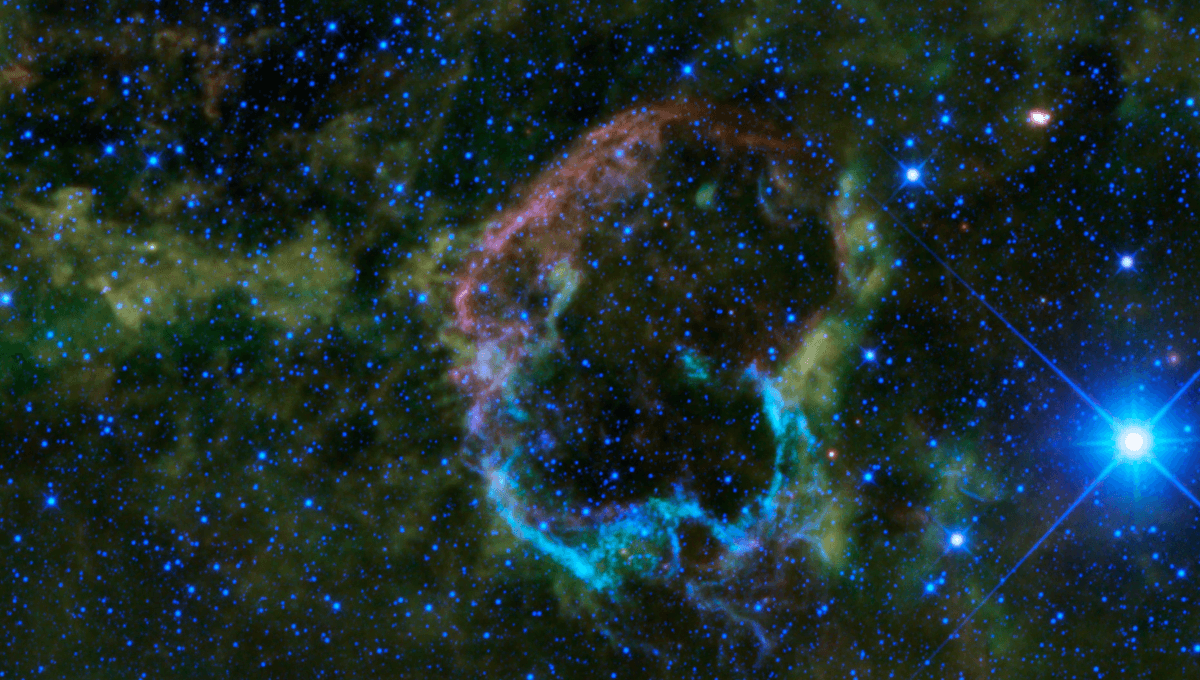
There are a small number of isotopes that offer clues regarding ancient supernovae that helped shape the Earth and the rest of the Solar System. Of these, the one astronomers and planetary scientists turn to most often is iron-60, so what makes it so useful?
Most of the elements that make up the Earth are formed in catastrophic explosions, either supernovae or kilonovae. Based on this fact alone we know that at least one, and probably several, such explosions occurred somewhere in our region of the galaxy before the Sun formed.
These explosions dispersed elements into the cloud of gas from which the Solar System condensed. Although the vast majority of that cloud was hydrogen and helium, various processes led to those two elements being rare on Earth, while concentrating most of the others.
The origins of iron
These initial elements consisted of a mix of stable and radioactive isotopes. In the case of iron, for example, the Earth inherited a great deal of iron-56 (that is atoms with 26 protons and 30 neutrons). Much smaller amounts of iron-54 and 57 (still with 26 protons, or it wouldn’t be iron, but 28 and 31 neutrons) and a tiny amount of iron-58 were also present. All of these are not radioactive, so the Earth has as much today as it ever did, allowing for some deliveries from meteorites.
However, the supernovae that seeded the cloud from which we sprung would also have created some iron-55, 59, and 60, all of which are radioactive. However, these isotopes took a very different path.
Iron-59 has a half-life of 44.6 days. A year after the explosion in which these atoms were formed, less than 1 percent survived. If any survived the journey from the exploding star to the Sun’s protoplanetary disk, it was gone by the time there was an actual planet. Iron-55 has a half-life of 2.7 years, but that’s still far too short for detectable quantities to be left from before the Earth’s formation, or even from nearby explosions that have happened since.
Why Iron-60 is special
However, iron-60 is a different matter. Its half-life is 2.6 million years – long enough to create a lasting legacy, but short enough that we can differentiate it from isotopes that last forever. We can even detect the presence of iron-60 after it is gone. Beta decay turns iron-60 to cobalt-60, which then turns relatively quickly into nickel-60. The distribution of iron-60 in the early Solar System has been investigated by looking for the abundance of nickel-60.
Meanwhile, ocean sediments reveal several spikes in iron-60 over the last few tens of millions of years. The Earth has no way of producing this isotope (at least until humans built nuclear reactors), nor a process for concentrating it. Instead, these increases indicate something off-planet must have showered Earth with iron-60 atoms. The same process would have also sprayed plenty of other isotopes over us.
However, as with the iron associated with Earth’s formation, those isotopes with substantially shorter half-lives are long gone. Those that are stable are so abundant on the planet already that a little extra from space is not noticeable.
We have even confirmed the extraterrestrial nature of these iron-60 spikes by finding matching ones on the Moon.
However, while we may have a general idea that upsurges in iron-60 occurred 3.4-1.7 million and 8 million years ago, identifying the source is trickier. Supernovae are thought to be the most common source of iron-60, but kilonovae can produce plenty as well. The length of time of the most recent iron-60 spike has been attributed by some astronomers to two supernovae happening close enough together in time that their peaks overlap, but by others to a kilonova instead. Telling the difference is not easy.
There’s even a third option, albeit an unlikely one. So-called asymptotic giant branch (AGB) stars can produce many of the heavier elements once thought to be the preserve of exploding stars alone. They not only make a lot of iron-60, but shed it into space, along with aluminum-26.
As far as we know, AGB stars don’t disperse these elements anything like as widely as supernovae. Nevertheless, a close encounter with an AGB star could produce a spike in abundance, although the chances of such a close encounter recently are considered small.
A further question is whether all this iron-60 matters. Although it is radioactive, iron-60 decays so slowly that it is quite safe in the volumes detected. However, the observed spikes coincide with periods of planetary cooling. The iron-60 itself would not be responsible for that, but it is possible, although still very speculative, that accompanying cosmic rays from the same explosion could have sparked increased cloud cover that affected the global climate.
Source Link: Iron-60 Is Not Of This World, So Where Is It From?