If you look at a current version of the periodic table, it appears complete. Since 2010, the entire seventh period has been filled, but the eighth period has not been started. It’s easy to wonder if we have discovered, or created, every element possible. Even if this is not the case, one might wonder whether some future maximum exists, beyond which it is impossible to create further elements. The answer is probably not – but there are some interesting complications to that.
First, a little chemistry refresher. Elements are defined by the number of protons in their nucleus. Six protons and it’s carbon, 26 protons mean iron, 92 uranium. An element’s atomic number equals its proton count. All elements, other than hydrogen, need some neutrons to hold the protons together, but it’s the number of protons that matter. Indeed, in most cases, the nucleus will be either stable or fairly long-lasting with several different numbers of neutrons, creating different isotopes of the element. For example, there are five stable isotopes of nickel.
The periodic table was initially constructed from naturally occurring elements based on similarities in their chemistry, and filled as far as uranium. Realizing elements with more than 92 protons were not found in nature, scientists started trying to make them.
Synthetic elements
We’ve now made 26 of these “transuranic” elements. None of them are stable. The reason we don’t find them in nature is not that they were never produced; almost certainly many of them have been made in supernovae and kilonovae. However, all are sufficiently radioactive that any whose origins predate the Earth have long since decayed to other elements. Production by more recent events has been far too limited to introduce detectable quantities to the Solar System.
Producing the first transuranic elements proved relatively easy. Neptunium and plutonium were made by bombarding uranium with light nuclei so that some got captured to produce still heavier elements. Producing enough plutonium to make an atomic bomb may have been a major challenge during World War II, but as the process was refined it proved too easy for humanity’s good thereafter. With half-lives of hundreds of thousands or millions of years, neither element had survived from the Earth’s beginning, but once made there was plenty of time to study their properties.
The next elements along the seventh period, americium and curium, were made only a few years later by a similar process and without much greater difficulty. Their half-lives are shorter, but still easily long enough to build up stocks of either element for research. From the late 1940s to the 1960s, new elements were announced every few years, steadily stepping further along the table – and even making it to song.
Some were produced deliberately by bombarding heavy elements with light nuclei, others found in the fallout from nuclear weapons tests.
However, the further along period 7 one goes, the harder it has proven to make new elements, and the shorter their half-lives have turned out to be, although the pattern is not perfect. Roentgenium (atomic number 111) and Darmstadtium (110) both have their longest-lived isotopes measured in seconds, but Roentgenium lasts longer than Darmstadtium, despite being one step further along.
Once you get to Moscovium (115) even the longest half-life is measured in milliseconds. We can’t really study its chemistry, because even if we could make a lot of it, very little would be left in the time it took to conduct an experiment.
No new elements have been announced since 2010 when tennessine (117) was made, although names and official recognition of the most recent elements occurred in 2015/2016. Oganesson (118) produced earlier than tennessine, has an even shorter half-life at less than a millisecond, and only a handful of atoms have been made.
So, is this it?
Probably not. There is no theoretical reason why heavier elements should not be possible. The table’s periods reflect the elements’ chemistry, caused by electrons, rather than the physics of the nucleus, so there is nothing theoretically stopping us from starting another row. Nevertheless, given how hard it has proven to make the last few elements, we can expect it to be harder still to make more and observe them before decay. There’s no unbreakable limit like the speed of light, but the level of difficulty can be expected to keep increasing.
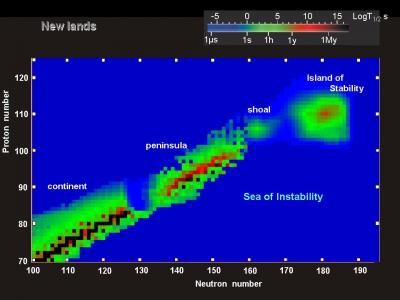
Plot Twist – A Possible Island Of Stability
More than 50 years ago physicists proposed that somewhere around atomic number 164 there could be an “island of stability”. More recently one of the main proponents has been Dr Yuri Oganessia, whose name might look rather familiar, reflecting the respect with which he is viewed by colleagues in the heavy element-making field.
Producing elements with such a large nucleus would still be difficult, to say the least. Yet if we can get there, the idea runs, they would last for long periods of time.
A prediction for the stablity of isotopes based on their numbers of protons and neutrons
Image Credit: Yuri Organessian
Recently, the idea got a kick-along from the fact that asteroids such as 33 Polyhmnia and Psyche appear to be very dense, astonishingly so in Polyhmnia’s case. It’s likely this apparent density just reflects measuring errors – we may be overestimating the mass and underestimating their volume.
A much more tantalizing explanation, however, is that these asteroids are built around cores of elements near the proposed island of instability. Elements’ density does not increase exactly in line with atomic number, but generally speaking, those with higher numbers are denser, and anything with an atomic number in the 160s should be staggeringly heavy.
If forged by the phenomenal force of a kilonova, such elements would be eternal, or nearly so. Small amounts could have made their way to our Solar System. Within planets, they would sink to the center and represent such a small part of the core as to be undetectable, but a decent lump could raise the average density of an asteroid to the point we notice something odd.
A more whimsical vision of the quest to find distant islands of stability.
Image Credit: Yuri Organessian
If this is true, and we could return a sample of such elements to Earth, it could form the basis for making elements with atomic numbers into the 170s. These might be beyond the island of stability, and therefore very short-lived, but could still be possible to produce very briefly.
Source Link: Is There A Maximum Number Of Elements In The Periodic Table?