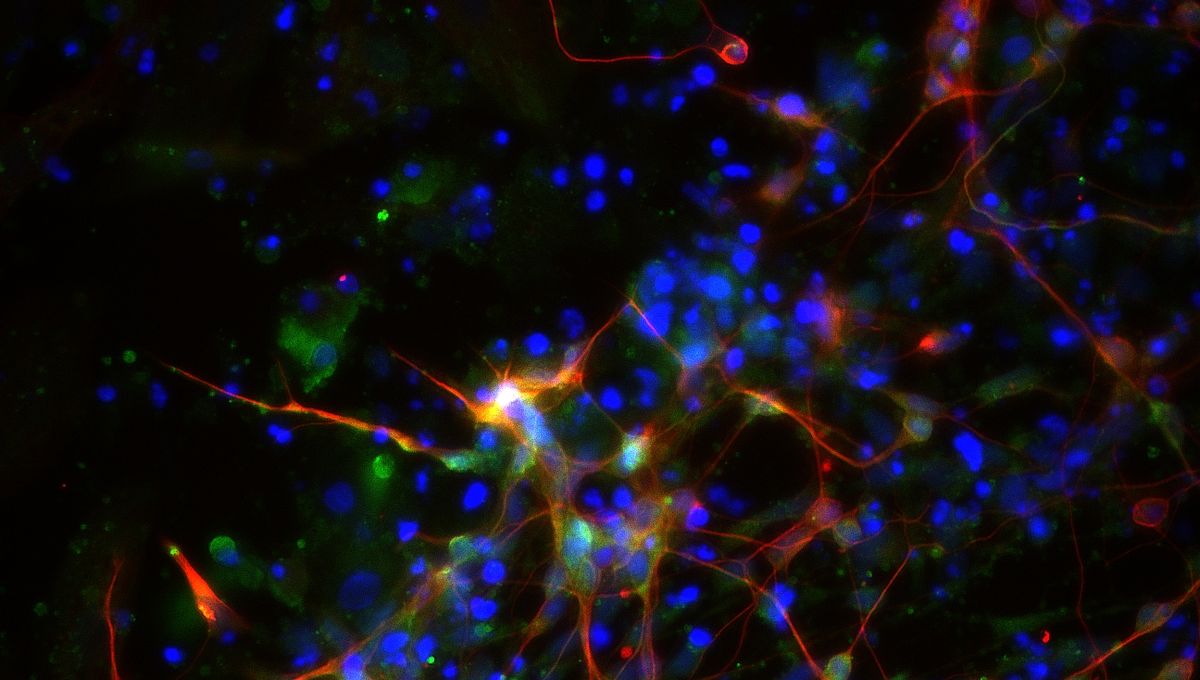
Neuroscientists in China have made a breakthrough in combating depression and other mental health disorders by growing dopamine neurons from human pluripotent stem cells that can target specific brain regions and repair mood-related circuits. The cells in question? A10 dopaminergic neurons. These cells are found in a part of the brain called the ventral tegmental area, which has an important role in feelings of reward and motivation.
Human pluripotent stem cells are cells with the ability to transform into any cell type. The scientists turned these versatile cells into A10-like dopaminergic neurons. The special formula was achieved by combining the stem cells with several factors, including Notch inhibitor (a compound that blocks the Notch signaling pathway), glial cell line-derived neurotrophic factor (a protein that boosts cell growth), and ascorbic acid (the fancy name for vitamin C), to guide the stem cells to transform into the desired neuron over 32 days.
This combination of ingredients worked together to encourage the stem cells to transform into A10-like dopaminergic neurons and to increase the number of cells produced. The A10-like dopaminergic neurons were then transplanted into anesthetized mice.
Not only did the lab-grown neurons look and behave like real A10 dopaminergic neurons, but when they were transplanted into mice, the neurons built connections with target specific regions and restored circuits responsible for mood and motivation. This resulted in an almost magical mood uplift where normal mice showed reduced anxiety and depressed mice exhibited antidepressant-like behaviors.
Basically, the transplanted neurons helped to repair the damaged brain circuits responsible for mood regulation in the mice.
Whilst this is the first time this specific approach has been used to treat depression, the idea isn’t entirely new. Back in 2020, scientists used human stem cells to generate pancreatic beta cells (cells that secrete insulin to regulate blood sugar levels) to treat mice with severe diabetes. This treatment successfully reduced the blood sugar levels within two weeks to a normal level, and blood sugar was regulated for at least nine months after treatment.
Similarly, neural stem cells have been used to repair damaged spinal cords in mice, where the implanted cells actually formed working connections with the host’s own neural network. Stem cell therapy is proving to be an amazing breakthrough across multiple conditions.
So why is this study important? Depression, specifically major depressive disorder (MDD), is the most common neuropsychiatric condition; patients experience anhedonia, the inability to feel pleasure or joy. MDD is estimated to rank as the third-highest contributor to the global disease burden by 2030. About a third of individuals suffering from MDD experience a treatment-resistant form, meaning their symptoms have not significantly improved despite undergoing standard treatments.
Although there are many types of approaches being tried to combat depression, it has been shown to be tricky to treat. New treatments including psychedelics such as psilocybin, a naturally occurring hallucinogenic compound, have faster antidepressant actions compared to the effects of current treatment. However, not all tested cases have shown compatibility. Sadly, in some isolated instances, these drugs have worsened depression symptoms after the treatment.
There’s also ketamine, famously known as the “horse-tranquilizer” anesthetic, which tackles depression by increasing calcium signaling in non-neuronal astrocyte cells, resulting in reduced signals to “give up”.
As shown, each of these treatments has its own target because the main cause of depression itself is subject to many different theories. Significant evidence has linked anhedonia to impairment in the dopamine reward pathways. Hence the stem cell transplant technique, which reconstructs and rebuilds broken neural circuits in the brain rather than adjusting chemical signals, may potentially offer a “significant advantage over systematically (or broadly) distributed pharmaceutical agents by providing much fewer off-target side effects,” according to the authors.
Whilst there is still a long journey from mice to medicine, this research shines light on the future of cell-based therapies for mental health disorders.
“This study provides proof-of-concept evidence supporting the use of cell therapy to treat psychiatric disorders by specifically reconstructing dysfunctional neural circuits,” explained the research team.
They have also considered the limitations of this study and suggested improvements for further development. In particular, the portion of generated A10-like neurons are in small quantities, meaning the recipe needs working on to boost their numbers and reduce off-target cells. Potentially, the researchers can also increase the activity of the A10-like neurons.
Of course, depression is not a simple mental health disorder, and animal models can only mimic a part of that complexity, meaning further tests across more animal models are required to check for its potential as a therapy for depression prior to human trials.
The study is published in Cell Stem Cell.
Source Link: Merry Mice: Human Brain Cells Transplanted Into Mice Reduce Anxiety And Depression