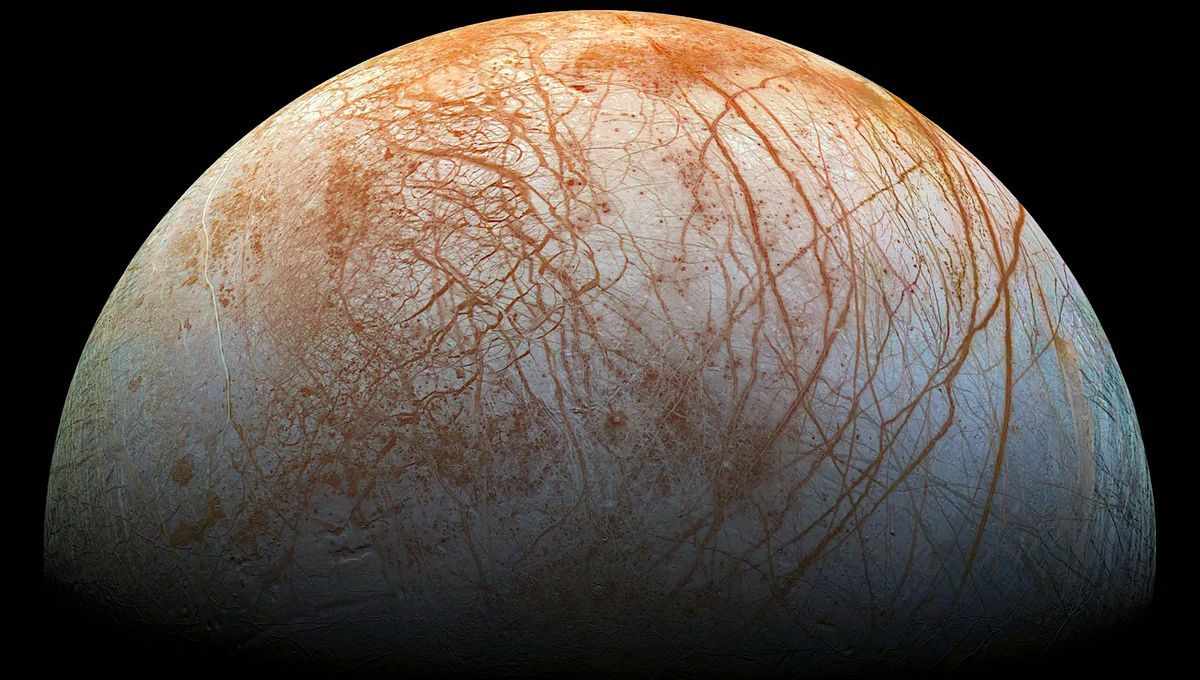
Researchers report two new types of ice made of salt water. The new ice can only form under high pressure and at cool temperatures but it can remain stable at low pressure. These new substances might be a component in the icy shells of moons such as Europa and Ganymede, and could also be present at the bottom of alien oceans.
The standard crystal that can form out of frozen salty water has a simple structure: Two water molecules per sodium chloride – the chemical name of the standard kitchen salt. But the two new hydrates – the technical name for these substances – have a lot more water than salt. One has 13 molecules of water per sodium chloride and the other one has a whopping 17.
“It’s rare nowadays to have fundamental discoveries in science,” lead author Baptiste Journaux, from the University of Washington, said in a statement. “Salt and water are very well known at Earth conditions. But beyond that, we’re totally in the dark. And now we have these planetary objects that probably have compounds that are very familiar to us, but in at very exotic conditions. We have to redo all the fundamental mineralogical science that people did in the 1800s, but at high pressure and low temperature. It is an exciting time.”
The red streaks on the surface of Europa are thought to be some peculiar salt but their chemical signatures are not something scientists are familiar with. These new ices might be what fits the bill.
“It has the structure that planetary scientists have been waiting for,” Journaux added.
The team did not set out to search for new ice. They were studying the way salt in water acts as an antifreeze and exploring the effect of pressure on that. It turns out that under pressure, new solid hydrates form, and one of the two (the one with 17 water molecules) remained solid even after the pressure was brought back to room standard.
“We were trying to measure how adding salt would change the amount of ice we could get, since salt acts as an antifreeze,” Journaux said. “Surprisingly, when we put the pressure on, what we saw is that these crystals that we were not expecting started growing. It was a very serendipitous discovery.”
“Pressure just gets the molecules closer together, so their interaction changes – that is the main engine for diversity in the crystal structures we found,” Journaux added. “We determined that it remains stable at standard pressure up to about minus 50°C (−58°F). So if you have a very briny lake, for example in Antarctica, that could be exposed to these temperatures, this newly discovered hydrate could be present there.”
The team plans to create more salty ice and study it in greater detail. The goal is to obtain chemical signatures and compare them with what spacecraft have measured on the distant frozen moons in the solar system.
New ice seems to be the thing at the moment, with a new structure of regular ice without salt announced just a few weeks ago.
This work is published in the Proceedings of the National Academy of Sciences.
Source Link: New Forms Of Salty Ice Discovered And They Could Be Covering Icy Moons