The temperature at which a material melts, subject to pressure, follows a single parabolic equation, one scientist has claimed, providing a surprisingly simple solution to a long-standing problem.
If you want to turn a solid to liquid the usual approach is to add heat. However, the temperature at which melting occurs depends not only on the substance involved but the pressure applied. Consequently, physicists and chemists use temperature-pressure phase diagrams, where the X-axis measures temperature and the Y-axis pressure, to show the conditions under which something will be solid, liquid, or gas. The melting line marks the boundary between the first two. Knowing where that line sits can be essential for choosing advanced engineering materials, and has applications in areas such as drug design.
These diagrams are usually produced by expensive trial and error – it would save a lot of money and heartache if there was a universal formula for predicting that line. That is what Professor Kostya Trachenko of Queen Mary University claims to have provided, although not everyone is entirely convinced.
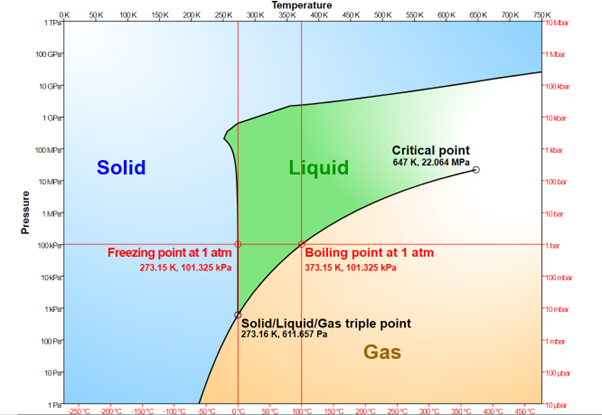
A phase diagram for water under different temperatures and pressures. The line from gas to solid and liquid is well understood, solid to liquid has to be pieced together experimentally.
Image Credit: Cmglee – Own work, CC BY-SA 3.0, via wikimedia commons
Trachenko has recently published a book called Theory of Liquids, and has used advances he and others have made on the topic to conclude melting lines can be described by a simple parabolic equation. Instead of needing to conduct experiments using ever-greater temperatures and pressures, with all the accompanying difficulties that involves, the line can be projected using measurements in more benign conditions, Trachenko argues.
Besides the practical value, such a discovery would also imply a fundamental commonality to apparently unrelated materials.
“The simplicity and universality of this result are particularly exciting,” Professor Trachenko said in a statement, although simplicity is a relative description here. “It suggests that melting, despite its complexities, exhibits a fundamental unity across diverse systems, from noble gases to metals.”
Trachenko derived the equation by focusing on the energy of a substance at the point where it melts or solidifies, rather than the entropy as most previous efforts have. He concludes the melting line is set by the Planck constant and the electron mass and charge, as well as the shells in which a substance’s outer electrons exist.
In one sense the existence of such a formula should not be a surprise. An equation for the point where liquids turn to gas is already known, based on theoretical thermodynamics. The same is true for sublimation – when solids turn directly to gas without a liquid stage, as occurs for comets approaching the Sun. On the other hand, given how heavily studied the topic is, finding something that has been missed until now is somewhat unexpected, particularly since the definition of the boundary between solid and liquid is not always straightforward.
Moreover, Dr Sergey Kharpak of the German Aerospace Center told New Scientist Trachenko used approximations based on “physical intuition” at several points in his derivation. Without rigorous proofs of these estimates, Kharpak said, only extensive testing under conditions that have not been used before will be sufficient to establish if Trachenko is right.
If ice queens and superhero sidekicks can freeze whatever they touch, there’s probably a market for fantasy villains to do the opposite but for that, you need to know where the line lies.
The paper is open access in Physical Review E.
Source Link: Physicist Claims To Have Worked Out How To Make Any Material Melt