Clinical trials are about to begin for a spinal injury treatment using a “bridge” – made of stem cells from the patients’ own noses – over the damage.
Millions of people worldwide have spinal injuries, creating immense demand for cures, so research indicating a path to treating spinal injuries using stem cells from the nose attracted major publicity. Now, the first participants have been enrolled in a clinical trial to test a more advanced version of the technology.
Earlier versions injected the cells at the site of the injury, but the Phase I/IIa trial will use 1-2 cm (0.4-0.8 inch) bridges built of millions of olfactory ensheathing nerve cells.
“Our innovative nerve bridges, combined with the high purity olfactory cells, offer what we think is the best hope for treating spinal cord injury,” Professor James St John of Griffith University said in a statement.
Spinal injuries are so hard to treat because the central nervous system’s cells do not regenerate in adults. Evolution has compensated for this vulnerability by encasing the components of the central nervous system in myelin sheaths, but this can present a further obstacle when the sheath itself is scarred.
Peripheral nerves retain the capacity to regenerate, but usually lack the features required to restore spinal injuries. Olfactory nerves are the exception. The olfactory ensheathing cells transplanted in this trial have a specialized role in the nose. Unlike other nerve cells, they regenerate every 6-8 weeks, an evolutionary response to the role they play in protecting the respiratory system against potential invaders such as bacteria. This makes their stem cells candidates for repurposing to replace other nerves that have been damaged.
It’s a dangerous life serving as a guard against intruders, so olfactory ensheathing cells have a short lifespan, forcing the body to produce more from stem cells that can differentiate into new cells. On the other hand, these cells also need to connect to the central nervous system to pass on their messages of detected scents to the brain. These twin capacities make them uniquely suited to spinal repair.
In 2002 a clinical trial showed that treating patients’ spinal injuries with stem cells harvested from patients’ own noses was safe. Many people believed that widespread treatment was around the corner – and certainly, headlines suggested as much.
“Since then, other trials around the world have also tested the cells but while there were some encouraging results, technical difficulties in preparing and transplanting the cells have been limiting factors.” St John said.
None of these trials have seen patients suddenly go from using wheelchairs to running marathons unaided, but ten years ago Darek Fidyka was able to walk while holding onto rails after a transplant from his own nose. Fidyka also regained some control over his bladder, bowels and sexual function that he had lost when a knife attack severed his spine, and after two more years of progress learned to ride a tricycle.
Nevertheless, success has not been reliable. St John said; “Despite decades of worldwide research to find a treatment for spinal cord injury, there is still no clinically available treatment.”
St John explained to IFLScience that this was partly because previous work either used cells collected from the olfactory bulb, which carries a substantial risk, or were nasal cells with purity as low as 11 percent. The team claims to have solved the problem of collecting pure nasal cells.
Perhaps more importantly, the early cells were injected in a liquid suspension, but were not in contact with each other. Fidyka’s trial; “Made 240 injections,” St John told IFLScience, risking causing damage in the process.
St John and colleagues in the team that performed the original safety trials developed the nerve bridges in response, and say they outperform other methods in animal trials. He told IFLScience their approach; “Has surgeons pick up the nerve bridge and place it over the scar and through the scar to the injury site. The cells have already made connections and are secreting growth factors to support each other before insertion.”
For rigor, a third of the participants in the trial will not receive the treatment. Instead, they will be given the same intensive rehab program those who have the surgery will get, which St John told IFLScience is not currently available in Australia. St John added that while no promises could be made, the program would look favorably on enrolling the participants not drawn to get the surgery for it later if the trial proves successful.
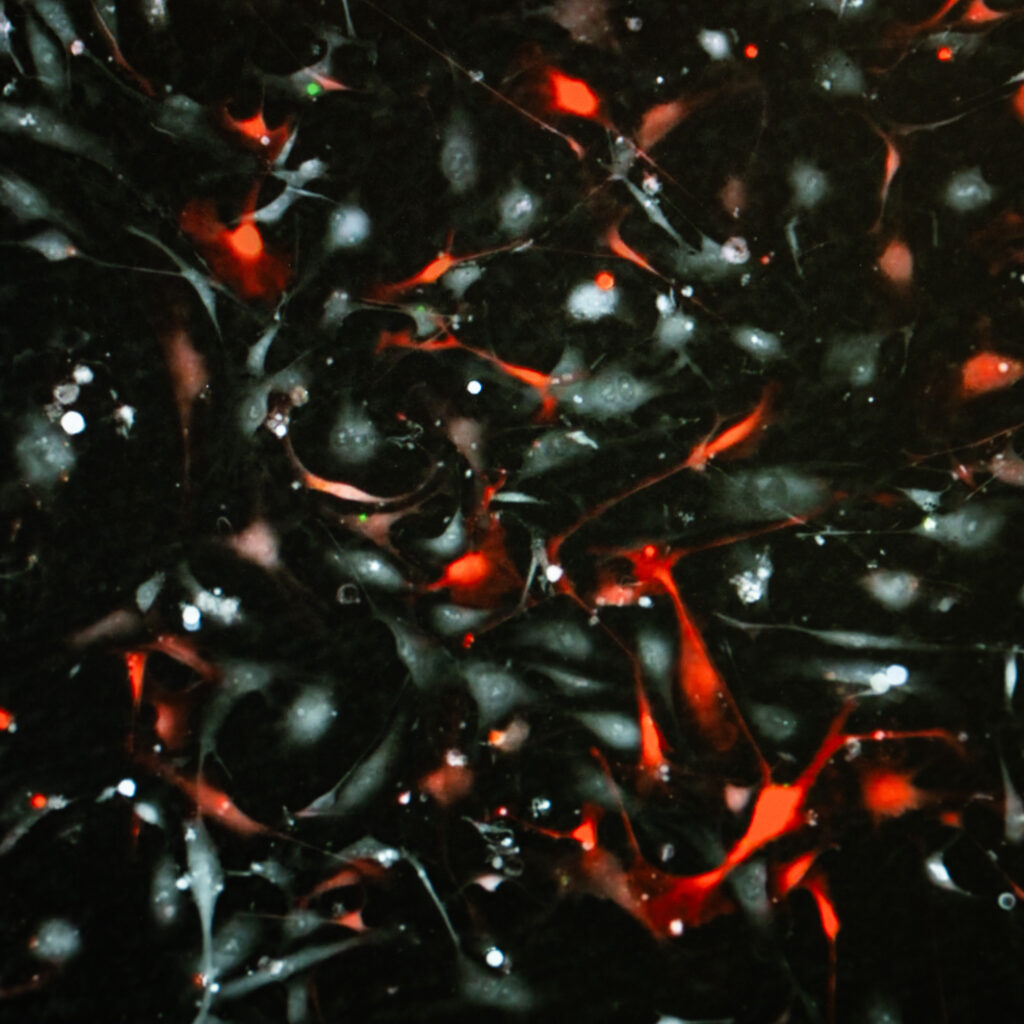
Olfactory ensheathing cells as seen under a microscope when not being molded into bridges.
Image Credit: Griffith University
There are hopes olfactory ensheathing cells could eventually provide even wider benefits, being used to treat brain injuries or neurodegenerative diseases. St John told IFLScience; “We’re focussed on the spine, although we are thinking about ways the approach could be used for peripheral nerve repair. Brain injuries are much more difficult.”
Many papers have been published on trials of olfactory ensheathing cells for spinal injuries, including by St John and his team, but the details of the bridge-building remain a closely guarded secret.
In addition to the problems of cell purity and survival, previous efforts stalled for lack of funding, which won’t come unless commercial sponsors expect to get a monopoly, at least at first. “It will be $50-$100 million for phase II clinical trials,” St John told IFLScience; “And phase three…” he trailed off. The combination of government and philanthropic money, plus support from the research institutions that have allowed this trial to go ahead, may not stretch that far.
St John and colleagues decided they needed to sacrifice transparency, and the career benefits of publishing, in order to maximise their chances of obtaining patents that could be the only way to bring the technology to market.
Trial places are still available: participants need to have lived with a spinal injury for at least 12 months – although they can register via [email protected] after four months – and live in south-eastern Australia. Further information on inclusion criteria can be found here.
Source Link: Revolutionary Nasal Nerve Cell "Bridges" For Treating Spinal Injuries To Begin Clinical Trials