A new type of ice is changing our understanding of water, as scientists discovered a frozen structure that more closely mirrors the molecular arrangement of a liquid, despite being a solid. The new ice type is amorphous, to use a polite phrase, which essentially means the organization of its molecules is a mess. Something to remember the next time you’re answering a Zoom call covered in string cheese: you’re not disorganized, just amorphous.
The discovery is a fascinating one as ordinary ice on Earth is very organized, becoming a solid by forming structured crystals with a rigid form. Finding the novel type of amorphous ice was, however, no accident, and actually came about thanks to some unique ball-in-a-cup play.
The scientific name for this game is ball milling, which for this study was used to shake ordinary ice and steel balls together in a frozen jar that was −200°C (−328°F).
“We shook the ice like crazy for a long time and destroyed the crystal structure,” said lead author Dr Alexander Rosu-Finsen, who carried out the experimental work while at UCL Chemistry, in a statement. “Rather than ending up with smaller pieces of ice, we realised that we had come up with an entirely new kind of thing, with some remarkable properties.”
The ball milling produced a previously unknown form of amorphous ice with disorganized molecules that had the same density as liquid water. As such, it’s been named medium-density amorphous ice (MDA).
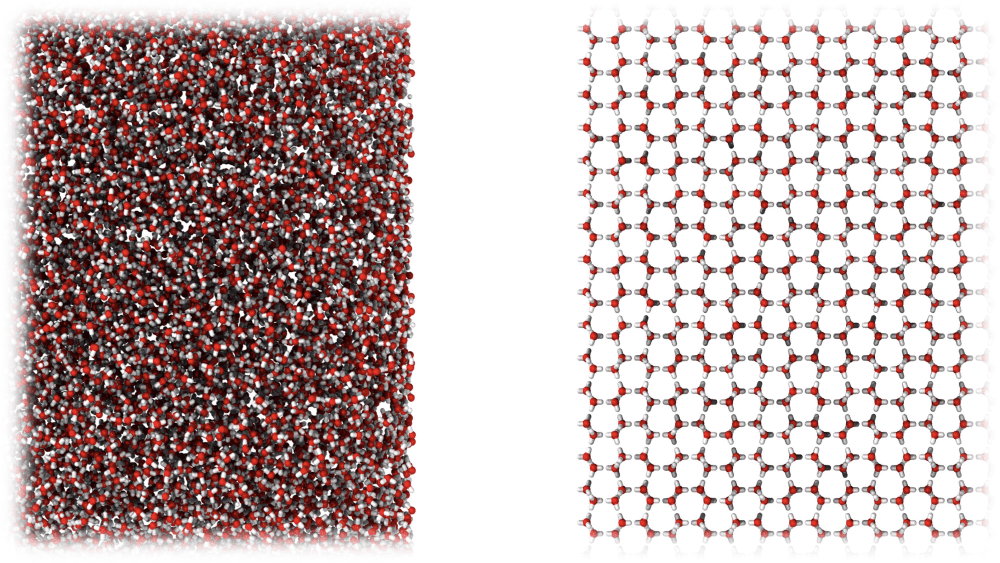
Our frozen mess MDA (left) and the organized structure of ordinary ice (right). Image credit: University of Cambridge
“We know of 20 crystalline forms of ice, but only two main types of amorphous ice have previously been discovered, known as high-density and low-density amorphous ices,” said senior author Professor Christoph Salzmann at UCL Chemistry.
“There is a huge density gap between them, and the accepted wisdom has been that no ice exists within that density gap. Our study shows that the density of MDA is precisely within this density gap and this finding may have far-reaching consequences for our understanding of liquid water and its many anomalies.”
MDA’s disorganized molecular structure means that it resembles a “stop-motion kind of water”, said co-author Professor Andrea Sella, also UCL Chemistry. “This is an unexpected and quite amazing finding.”
Its existence brings into question our understanding of liquid water, something the researchers say we actually still quite poorly understand despite chugging it on the daily.
“Water is the foundation of all life,” continued Salzmann. “Our existence depends on it, we launch space missions searching for it, yet from a scientific point of view it is poorly understood.”

Part of the set-up for creating medium-density amorphous ice. Image credit: Christoph Salzmann
“Existing models of water should be re-tested. They need to be able to explain the existence of medium-density amorphous ice. This could be the starting point for finally explaining liquid water.”
So, something of an alien when it comes to Earthly ice, and there’s even reason to believe it may exist out of this world. MDA could potentially be floating about inside ice moons of the outer solar system, suggest the researchers. This is because the gas giants Jupiter and Saturn could feasibly be exerting tidal forces that act on icy moons in a similar way to ball milling.
Another curious finding about MDA was that when it was warmed and recrystalized it released an enormous amount of heat. Such a reaction could give rise to “icequakes” on moons like Ganymede that are thickly covered as the ice breaks up and shifts in a similar way to the tectonic motion that creates earthquakes on our planet’s crusty surface.
A pivotal moment in our understanding of one of the most important chemical compounds on Earth, and one that holds exciting future possibilities for stop-motion slush puppies.
The study was published in Science.
Source Link: Scientists Have Discovered A New Type Of Ice, And It's A Mess