Fractals – those self-similar shapes which can be endlessly zoomed in on without losing detail – are weirdly ubiquitous in nature. You’ve got snowflakes, famously; cauliflowers and coastlines; even rabbits, with a bit of work, can be shown to conform to fractal patterns.
Of course, none of these examples can truly be said to be fractal outside of the math classroom – the real world simply doesn’t work that way. Whilst for mathematicians, zooming into infinity and its reciprocal just requires taking a couple of limits, the rest of us will eventually have to deal with things like “atoms” – and the consequences of trying to go smaller than that.
Which raises a question: how small can we actually go? And according to a recent discovery led by researchers at the Max Planck Institute in Marburg, Germany, the answer is… almost all the way.
“All known regular fractals in nature are made by living organisms and exist at the macroscopic scale. However, none have yet been discovered in nature at the molecular scale,” explains the team in a new paper describing the find.
But that’s all changed now: “We report the discovery of a natural metabolic enzyme capable of forming Sierpiński triangles in dilute aqueous solution at room temperature,” the paper reports.
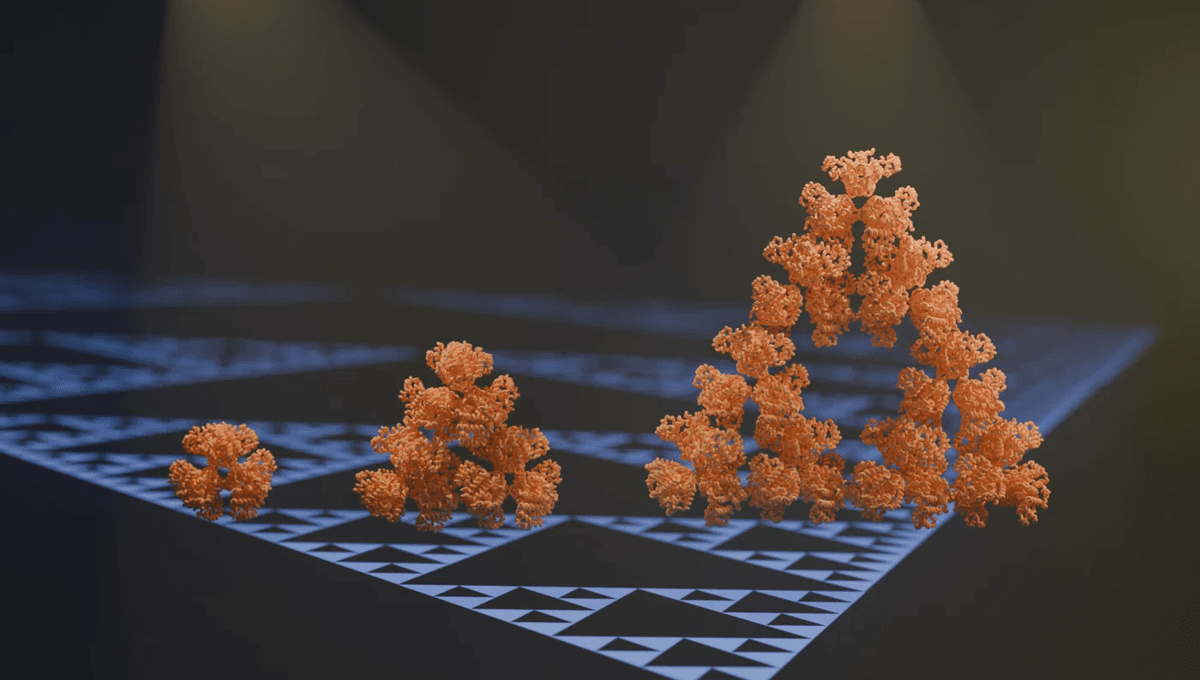
In other words, they’ve discovered the first-ever naturally occurring fractal molecule.
“We stumbled on this structure completely by accident,” first author Franziska Sendker said in a statement on the find. “[We] almost couldn’t believe what we saw when we first took images of it using an electron microscope.”
“The protein makes these beautiful triangles,” she explained, “and as the fractal grows, we see these larger and larger triangular voids in the middle of them, which is totally unlike any protein assembly we’ve ever seen before.”
So what makes this molecule so different from all the others? With the help of electron microscopy, the team was eventually able to decipher its structure – and what they found defied expectations for how proteins can assemble.
To put it simply, this particular molecule – a citrate synthase from the cyanobacterium Synechococcus elongatus – just isn’t as particular as normal proteins. Instead of building itself up symmetrically, with each individual protein chain being identical in arrangement to its neighbors, S. elongatus assembled itself slightly wonkily. The result? A Sierpiński triangle structure, holding up even as the proteins grow larger.
“This was one of the harder, but also more fascinating structures I have solved in my career,” said Jan Schuller, a researcher at Philipps University Marburg, whose group helped determine the structure.
“The problem with determining the structure of a fractal is that our image averaging techniques kept getting confused by the fact that the smaller triangles can be substructures of larger triangles,” he explained. “The algorithm kept homing in on these smaller triangles instead of seeing the larger structures they were part of.”
And the best part? It seems like this so-far unique molecular structure might have arisen completely by accident.
“[W]e can never be totally sure of the reasons why things happened in the past,” said Georg Hochberg, an evolutionary biologist and senior author of the study. But, he explained, “this particular case does have all the trappings of a seemingly complex biological structure that just popped into existence for no good reason at all because it was simply very easy to evolve.”
Which is exciting, to say the least. Because let’s face it: if molecular-scale Sierpiński triangles have been around us all along, popping up basically randomly just because they could – then what else might be out there, just waiting to be discovered next?
The study is published in the journal Nature.
Source Link: Scientists Just Discovered The First-Ever Fractal Molecule In Nature