A new method for making diamonds bypasses the high temperatures and pressures, opening the door to making them at a fraction of the existing cost. The world of fine crystal control called The Diamond Age in science fiction may be closer than we think.
Although we have known how to make synthetic diamonds since the 1950s, the dominant process requires temperatures of 1,300-1,600 °C (2,400-2,900 °F) and 50,000 atmospheres of pressure over 5-12 days. This has helped meet the industrial demand for diamonds as cutting instruments, as well as providing colors rare in nature for those whose tastes run that way. However, the cost of the process is close enough to that of finding natural diamonds, whether for industrial purposes or clear gemstones, that the mining industry survives.
That could be about to change with the announcement of a way to make diamonds at ordinary atmospheric pressure. The temperatures are still high – 1,025 °C (1,877 °F) – but even that means big savings compared to the heat currently required.
Low-pressure diamonds were thought to be a contradiction in terms. Natural diamonds are made in the Earth’s mantle with the force of kilometers of crust bearing down, and most predate multicellular life. The synthetic version uses liquid metal catalysts, but Gigapascal range pressures have still been considered essential.
However, researchers at Korea’s Institute for Basic Science have thrown that out, showing that a liquid metal alloy of gallium, iron, nickel, and silicon can grow diamonds without much pressure in a hydrogen/methane atmosphere. The methane provides the carbon from which the diamond grows.
“This pioneering breakthrough was the result of human ingenuity, unremitting efforts, and the concerted cooperation of many collaborators,” said Professor Rod Ruoff in a statement. He left out a lot of trial and error, which a team at the Institute used when adjusting the mix of metals and other parameters. Although making the diamond itself turns out to be a surprisingly quick process, it was only when the team shifted to a smaller chamber, which took less than one-twelfth the time to prepare, that real progress was made.
Eventually, it was found that when the liquid alloy is 77.75 percent gallium by atomic abundance, 0.25 percent silicon, and 11 percent each of iron and nickel, the diamonds grow near the bottom of the liquid. It’s not a ratio that immediately springs to mind. Moreover, unlike conventional synthetic diamonds, seed particles are not required.
“One day […] when I ran the experiment and then cooled down the graphite crucible to solidify the liquid metal, and removed the solidified liquid metal piece, I noticed a ‘rainbow pattern’ spread over a few millimeters on the bottom surface of this piece,” said graduate student Yan Gong. “We found out that the rainbow colors were due to diamonds!”
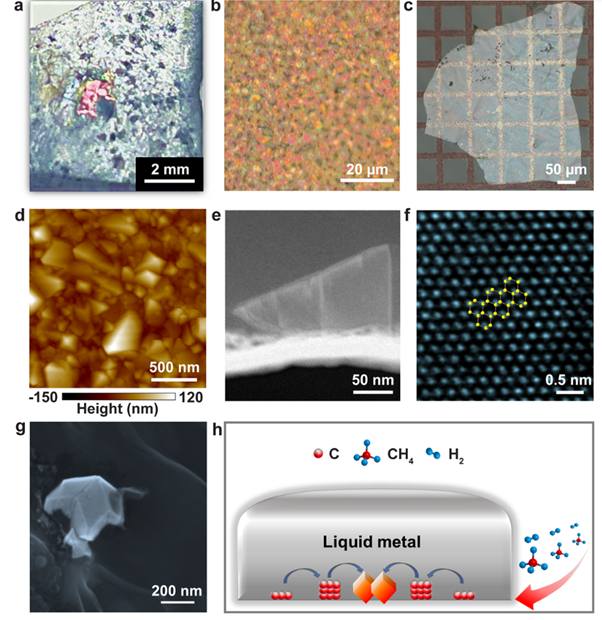
Diamond growth as seen at a variety of scales and using different instruments (a-g) and a schematic of the process.
Image Credit: Gong et al/Nature
The process takes between 10 and 15 minutes to start forming diamonds, and growth stops by 150 minutes, although the team hopes to find ways to overcome this.
The diamonds created so far are small enough – more a film than a gemstone – that diamond companies don’t need to panic quite yet. That could change, however, if methods are found to promote the supersaturated carbon layer that precedes diamond formation. The silicon-vacancy prized for producing colored diamonds, also produced by nitrogen impurities, could make the products ideal for experiments in quantum computing.
Why this combination of metals and gases gives the desired outcome, is still not fully understood. It is thought the similarity of silicon and carbon bonds may be key, with carbon clusters containing silicon atoms potentially serving as diamond precursors.
Mass production seldom ends up relying on the first version of a process demonstrated in a lab. Ruoff suggests a variety of lower melting point metals might prove useful, either to make the process cheaper still or to produce doped diamonds of particular shades or properties.
The study is published in the journal Nature.
Source Link: Synthetic Diamonds Made In Minutes Not Days Could Upend Gemstone Economics