Scientists are saying they’ve made a “critical first step” in developing a treatment that could allow people to regenerate their lost or broken teeth. With further work, they hope this breakthrough could be used to make “living filings” that grow and produce real enamel to regrow the tooth.
In a new study, researchers from the University of Washington created an organoid in a lab that was capable of producing the proteins that form dental enamel, the outer protective layer of teeth (and the hardest material in the human body).
This was achieved with the help of stem cells that were coaxed into becoming the specialized cells, called ameloblasts, that make enamel during tooth formation. These cells die off after tooth formation is complete, leaving the adult body with no way of regenerating the much-needed enamel.
Now though, scientists have managed to create ameloblasts in the lab, offering a potential way to overcome this problem.
“This is a critical first step to our long-term goal to develop stem cell-based treatments to repair damaged teeth and regenerate those that are lost,” Hai Zhang, study co-author and professor of restorative dentistry at the University of Washington, said in a statement.
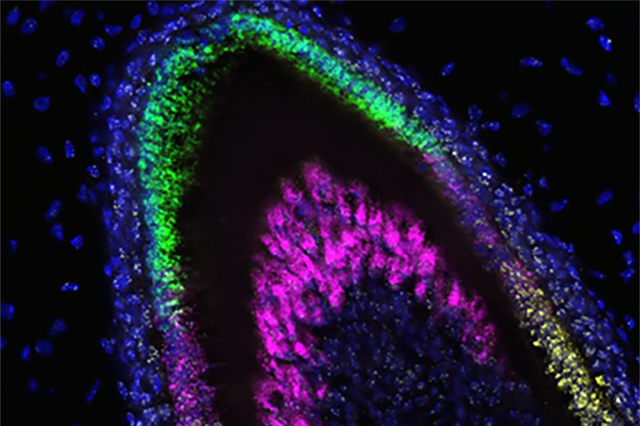
The different colors in this image of developing enamel show which genes are being expressed at each stage of development.
Image credit: UW Dental Organoid Research Team
To obtain the “blueprint” of how to make ameloblasts, the team had to look at the fiddly genetics that underpins cell generation in the body. DNA is like a recipe book that holds the instructions for making all the proteins in our bodies. These instructions are delivered to the molecular machines that assemble proteins through RNA molecules, called messenger RNA (mRNA).
Throughout the numerous stages of any tissue’s development, different proteins are needed at each stage, which is regulated through the turning on and off of genes. To recover the stage of enamel production, the team used a technique called “single-cell combinatorial indexing RNA sequencing (sci-RNA-seq).” A computer model was then used to understand how the pattern of gene activity manages to code for proteins that turn undifferentiated stem cells into fully differentiated ameloblasts.
The end product is a complex organoid: a tiny, three-dimensional, multicellular mini organ in a Petri dish.
This could potentially lead to the development of so-called “living fillings” that could grow and repair cavities and other defects, explains Professor Hannele Ruohola-Baker, a world-leading expert in regenerative medicine at the University of Washington, who led the project.
“Many of the organs we would like to be able to replace, like human pancreas, kidney, and brain, are large and complex. Regenerating them safely from stem cells will take time,” Ruohola-Baker said. “Teeth on the other hand are much smaller and less complex. They’re perhaps the low-hanging fruit. It may take a while before we can regenerate them, but we can now see the steps we need to get there.”
A number of studies have recently boasted promising results in the field of tooth regeneration. One notable example is the work of scientists from Kyoto University who showed how a protein called USAG-1 limits the growth of teeth in mice. By turning off the gene that codes for the production of the protein, the mice were able to freely regrow their teeth. While the research from Japan is also in its early days, it’s been suggested that human clinical trials could be started as soon as 2024.
The study is published in the journal Developmental Cell.
Source Link: Tooth Regeneration Breakthrough Could Lead To "Living Fillings"