Using advanced telescopes, scientists have looked at light that left galaxies 13.5 billion light-years ago, from the distant galaxy JADES-GS-z14-0. So why do we have such trouble looking the other way, down to the tiniest scales? Can’t we use lenses to see atoms?
ADVERTISEMENT GO AD FREE
The short answer is; no. We will never see atoms using visible light, simply because the wavelength of visible light (around 400 to 700 nanometers) is larger than the size of an atom (around 0.1 to 0.3 nanometers). Think of how a pixel on your screen cannot be used to display an object smaller than a pixel.
“It never will be possible to see atoms or molecules using visible light, even with the most powerful of microscopes,” Purdue University explains.
“In order to see an object, its size has to be at least half the wavelength of the light being used to see it. But the wavelength of visible light, though small, is much bigger than an atom, making it invisible. X-rays, however, have a wavelength short enough that they can be used to ‘see’ atoms.”
X-rays have a wavelength of around 0.01 to 10 nanometers, making them small enough to be useful in imaging atoms.
“When X-rays hit a crystallized molecule, the electrons surrounding each atom bend, or diffract, the X-ray beam, which then forms a pattern as it exits — an X-ray diffraction pattern,” Purdue University continues. “Crystals are used because the diffraction pattern from one single molecule could be insignificant, but the many individual, identical molecules in a crystal amplify the pattern.”
Using this, and refining the techniques, scientists have been able to create images of even single atoms.
ADVERTISEMENT GO AD FREE
Electrons are also small enough to be used to image atoms and molecules, using a variety of techniques in electron microscopes. Size isn’t everything, but fortunately, electrons possess another property; a wavelike nature.
“The wave-particle duality of electrons forms the foundation of electron microscopy. By treating electrons as waves, scientists can harness their short wavelengths to create detailed images of atoms,” Electron Beam Machine explains. “This duality also explains why electron beams are so effective for high-resolution imaging. The ability to switch between particle and wave behavior gives electrons unique advantages in studying the smallest building blocks of matter.”
Scientists send beams of electrons at a sample at high speeds, and then detect where they (and other resulting rays) end up.
ADVERTISEMENT GO AD FREE
“As the beam passes through or scans across a sample, electrons interact with the atoms in various ways. These interactions produce signals, such as scattered electrons or emitted X-rays, which scientists use to construct images. The electron beam essentially ‘illuminates’ the atomic structure, allowing you to see features that are invisible to the naked eye.”
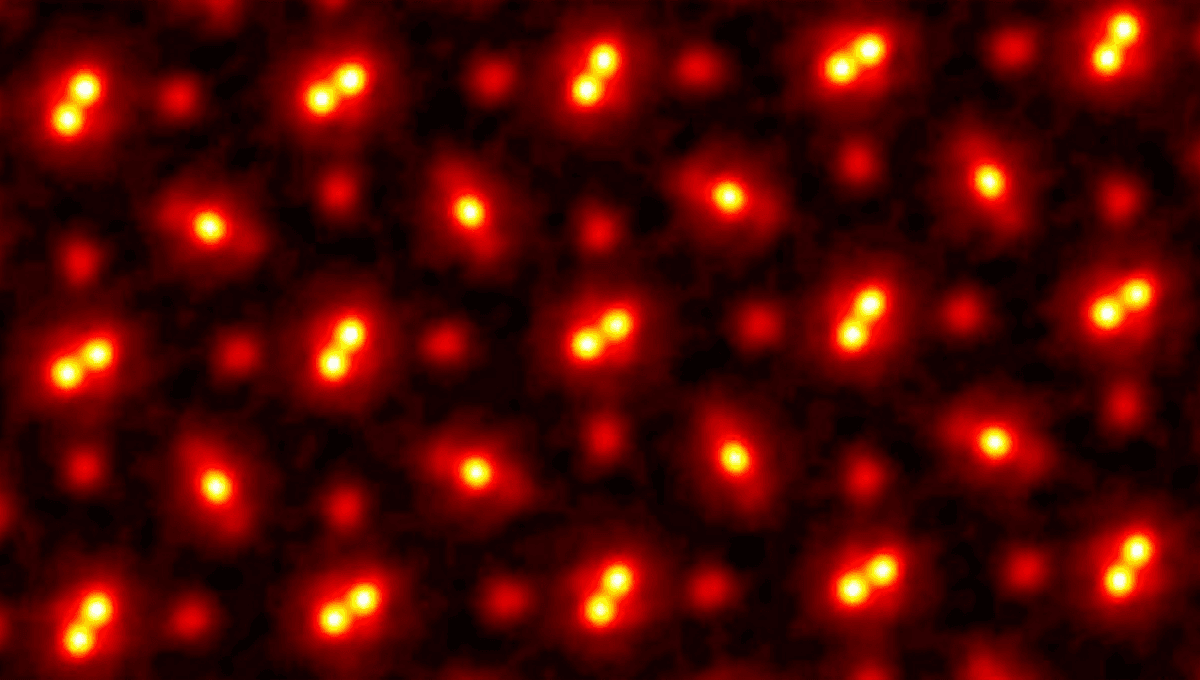
Scientists have gotten very good at using this technique to view the small world. In recent years, Cornell University researchers combined electron scanning with ptychography – scanning layers of overlapping scattering patterns from a sample – to produce the world’s highest resolution image of atoms. The image (seen at the top) was taken of a praseodymium orthoscandate (PrScO3) crystal, magnified 100 million times.
While impressive, barring some new breakthrough, we might not be able to see atoms in any better resolution.
ADVERTISEMENT GO AD FREE
“This doesn’t just set a new record,” David Muller, Professor of Engineering at Cornell, said in a 2021 statement. “It’s reached a regime which is effectively going to be an ultimate limit for resolution. We basically can now figure out where the atoms are in a very easy way. This opens up a whole lot of new measurement possibilities of things we’ve wanted to do for a very long time. It also solves a long-standing problem – undoing the multiple scattering of the beam in the sample, which Hans Bethe laid out in 1928 – that has blocked us from doing this in the past.”
While there are smaller particles than electrons, they can’t really be used for imaging. Neutrinos, for instance, only interact via the weak force and gravity. We have enough trouble detecting them; they are not going to be great for imaging the very small. Scientists will need to come up with some new clever imaging techniques to see any smaller, or else we may have already seen the parts that make us in the highest resolution we are ever going to get.
Source Link: What Is The Best Image We Have Of Atoms?