The science of super glue is nothing like watching paint dry. Within this situation-saving liquid, there’s some fascinating yet relatively simple chemistry going on.
Like many other good discoveries, super glue was invented by accident. The person credited with its creation is Dr Harry Coover, a chemist from the US who was attempting to make a clear material that could be used to make precision gunsights for soldiers during World War Two. The super-sticky chemicals they were working with proved to be useless for making gunsights, but Dr Coover later came to realize they had the potential to be a powerful adhesive.
Chemicals known as cyanoacrylates are the star of the show in super glue. In particular, most super glues you buy in the shops are primarily made of ethyl 2-cyanoacrylate. They also contain smaller amounts of thickeners, stabilizers, and preservatives.
When kept in its air-tight bottle, the chemical is made up of monomers, singular molecules that are like separate links in a chain yet to be bound. That all changes in the presence of water, which sparks a chemical reaction that binds the links together in a chain at an atomic level.
The chain polymerization reaction occurs due to trace amounts of hydroxyl ions (OH-) in water, which allow the separated molecules of ethyl 2-cyanoacrylate, aka monomers, to link together into long chains, known as polymers. The atomic bonds that make up these chains are tough and extremely difficult to break once formed.
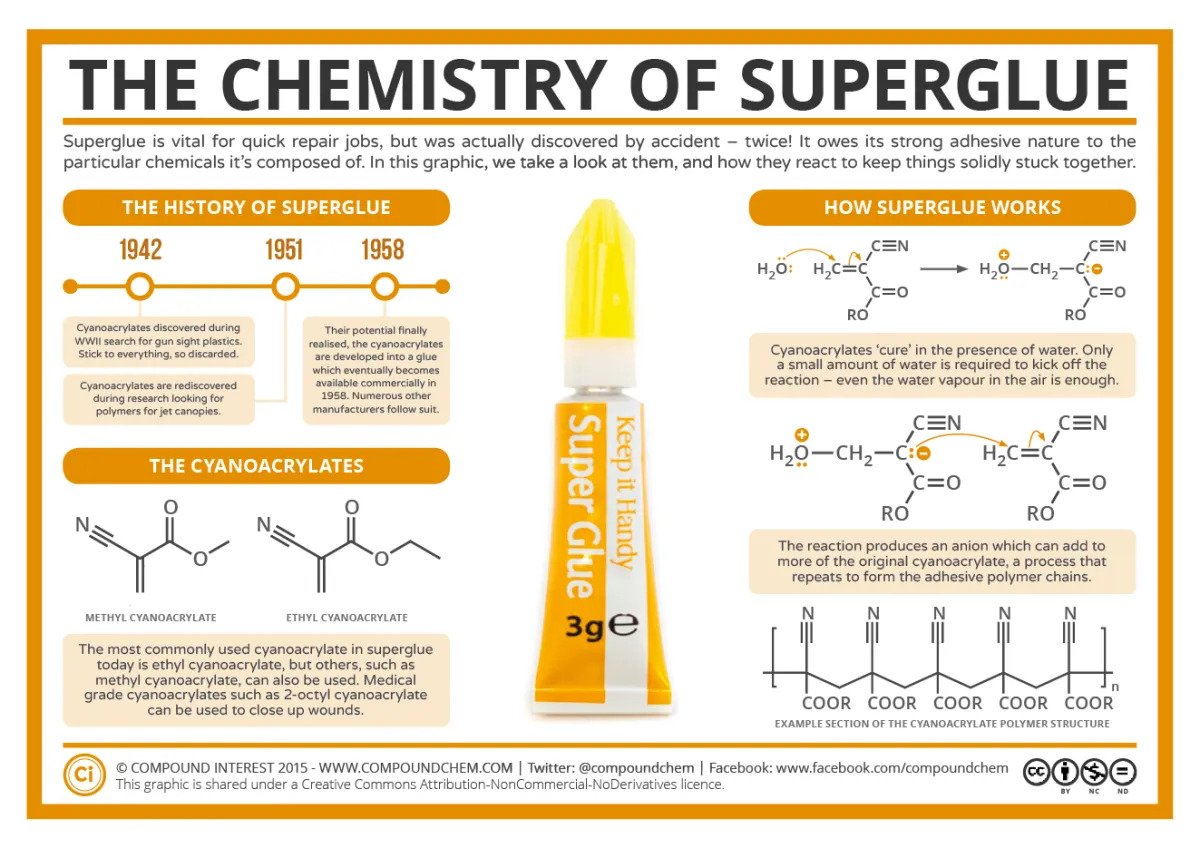
The science of super glue.
Since there’s humidity present in the air, as well as tiny amounts of water on most objects you’d want to stick together, the super glue will polymerize and harden when it’s released from the bottle and introduced to its new environment.
As a semi-viscous liquid, the super glue will seep into all the microscopic nooks and crannies of the two objects being glued. Post-polymerization, the glue sets and turns into a deeply interconnected solid, sticking the two bits together like, well, glue.
How to get super glue off skin
If you’ve ever found yourself with your fingers stuck together, that’s not too surprising since the ethyl 2-cyanoacrylate will rapidly polymerize in the presence of moisture and oils on your hands.
Fortunately, there is a way to weaken super glue’s powers once set. Acetone, one of the components of nail polish remover, is a solvent that will dissolve the super glue substance.
Bear in mind, it will still remain as a polymer – those bonds it made are incredibly strong, after all – but its dissolving action should be enough to free your fingertips. Pure acetone dehydrates the skin, however, so use it sparingly and with caution.
For the hardcore chemistry heads among you, a slightly more detailed explanation of this process is explained by Brent Amberger, who has a PhD in Organic Chemistry, of Goobertown Hobbies in the video above.
Source Link: What Makes Super Glue So Damn Sticky?