While most of the world measures temperatures in Celsius and the United States clings to Fahrenheit as if its hands were frozen, scientists usually prefer Kelvin. A degree Kelvin marks the same difference in temperature as a degree Celsius, but the starting point is 273.15 degrees lower. If you think that sounds like an odd number, it’s used because that puts 0 Kelvin at absolute zero, the point where there is no heat at all.
Yet nothing in the universe, neither the cold of outer space nor anything humans have produced, is at absolute zero. Unless there is something substantially wrong without our understanding of physics, nothing ever will be 0 K either, although we have achieved temperatures of 0.00000000004 K. So why is absolute zero impossible?
What does temperature really measure?
Energy causes atoms and molecules to move around, vibrating in a solid or bouncing around more erratically in liquids or gases. The more average energy a substance has, the faster the molecules move and the higher its temperature.
As temperatures drop towards zero Kelvin, that movement slows to a crawl. We might expect that just extracting a little more heat would lead movement to stop entirely, and we would be at absolute zero.
However, the third law of thermodynamics (tired of being overshadowed by its more famous sibling), would like a word.
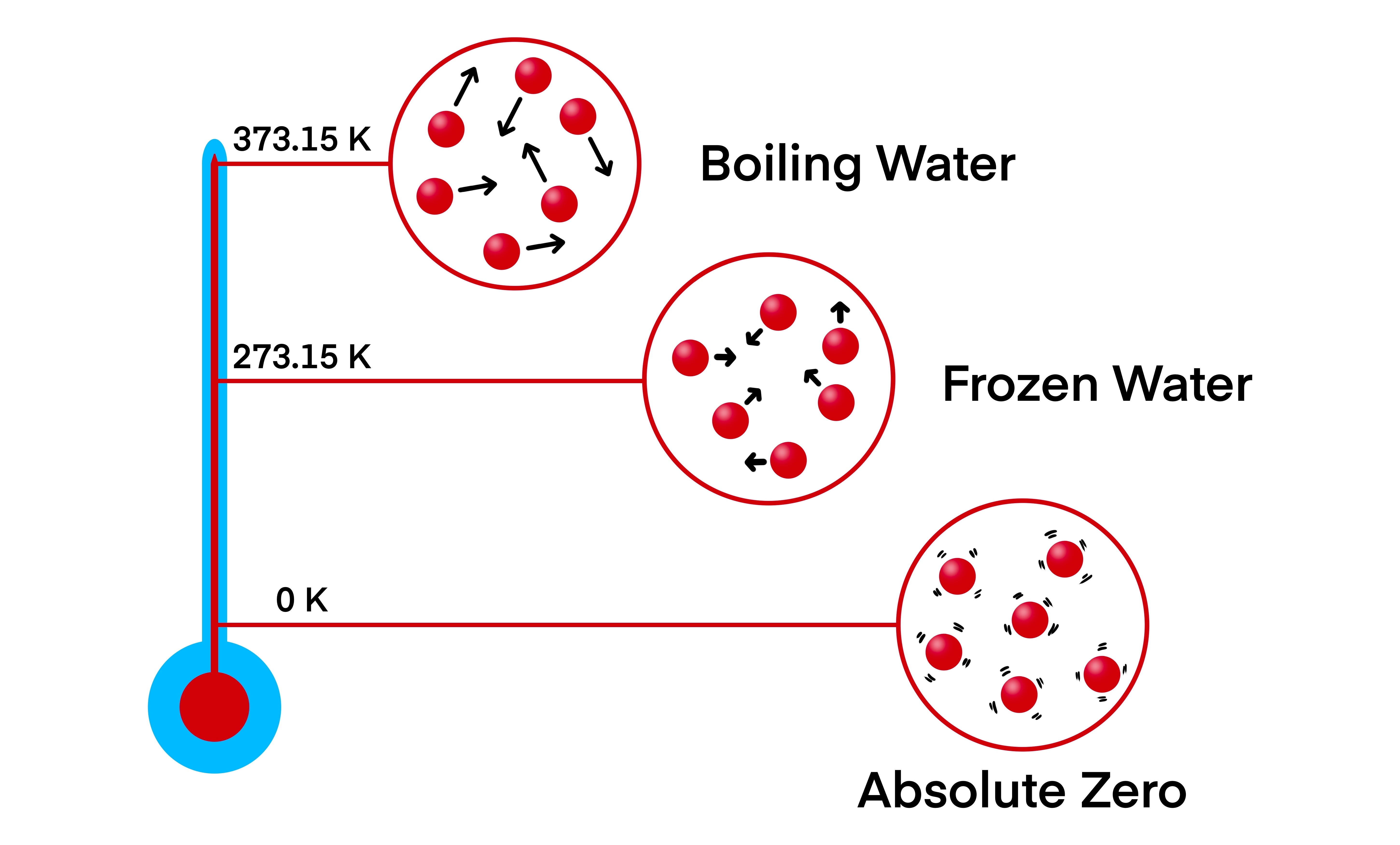
The motion of particles at different points on the Kelvin scale.
Image credit: Watthana Tirahimonchan/Shutterstock.com
An infinite road
As with the first two laws of thermodynamics, the third law can be formulated in many ways. The original may be the easiest to understand. Walther Nernst presented his law by saying, “It is impossible for any procedure to lead to the isotherm T=0 in a finite number of steps.” In other words, to reach absolute zero, energy must be removed from a system an infinite number of times. That, of course, cannot be done.
Nernst reached this conclusion through experiments, where he successively cooled substances, but always had some heat left to remove. No cooling process could take all the heat out of a sample in a single go, and the heat left behind kept the temperature above absolute zero. Statistical mechanics subsequently proved that this conclusion can be derived from the other laws of thermodynamics.
More recently, scientists showed it is also impossible to reach absolute zero in a finite amount of time, so it would take an infinitely old universe for absolute zero to exist.
How do we get near absolute zero?
If you want to get temperatures in your freezer/icebox to 0 °C, you move the heat to the outside world using a refrigeration cycle. Gas is compressed, heating it up. Much of this heat is drawn off to the surroundings. The gas is then allowed to expand, cooling it, in isolation so that it can’t heat up again. The product can then be passed near whatever needs cooling, drawing the heat out.
Repeated often enough, the process will cool helium to -269 °C, or just 4 degrees above absolute zero. A substance immersed in enough liquid helium will release its heat until it reaches the same temperature.
This approach allows us to achieve temperatures not much higher than the cosmic background radiation, which at 2.7 K, left over from the Big Bang, is the temperature of the universe where nothing warms it. We can go a degree lower using helium–3, the rarer isotope with just one neutron.
If we want to get colder still, things get harder. Processes like nuclear demagnetization, where magnetic fields are used instead of changes in pressure, alternately aligning the magnetic poles of atomic nuclei and releasing them, are sometimes used.
Laser cooling allows us to bring microscopic amounts of material to temperatures less than a billionth of a degree above absolute zero. The process, which won the 1997 Nobel Prize for Physics, has lasers focused in three dimensions on a cloud of atoms to resist their motion in any direction. The lasers act like a frictional force, slowing the atoms down and therefore making them lose energy.
Effective as this is, it doesn’t get around the third law; there is still some heat left behind when the lasers have done their thing. A more exotic approach, known as a matter-wave lens, has cooled rubidium atoms to temperatures 10 times lower still. This is great if you want to study Bose-Einstein condensates in brief moments of microgravity, but still won’t get you to absolute zero, unless you pretend by rounding off at ten decimal places.
While you’re here: Don’t be fooled by “negative temperatures”
Physicists sometimes refer to “negative temperatures”, which sound as if they are colder than absolute zero. However, the name is misleading. These are systems where any extra energy added decreases the entropy, or disorganization, of the system, instead of increasing it as is normally the case. For this to happen, the system needs to have a maximum amount of energy it can hold, something that requires quite unusual conditions. However, these systems are not super-cold, let alone having less than zero heat; in fact, they are hot enough that when exposed to a normal environment, the heat will flow out of the “negative temperature” system to its surroundings.
Source Link: Why Can’t We Reach Absolute Zero?