All that is gold is not gold-colored, as Bilbo Baggins probably meant to say. Sometimes it’s purple, as in the Alhambra Palace where a gold layer on wall and ceiling ornaments has changed color over centuries. The chemistry of this conversion has finally been explained.
The Alhambra Palace in Granada is considered one of the greatest examples of Islamic architecture in the world. Construction started in 1238, but the palace was modified and expanded many times, particularly in the 14th Century.
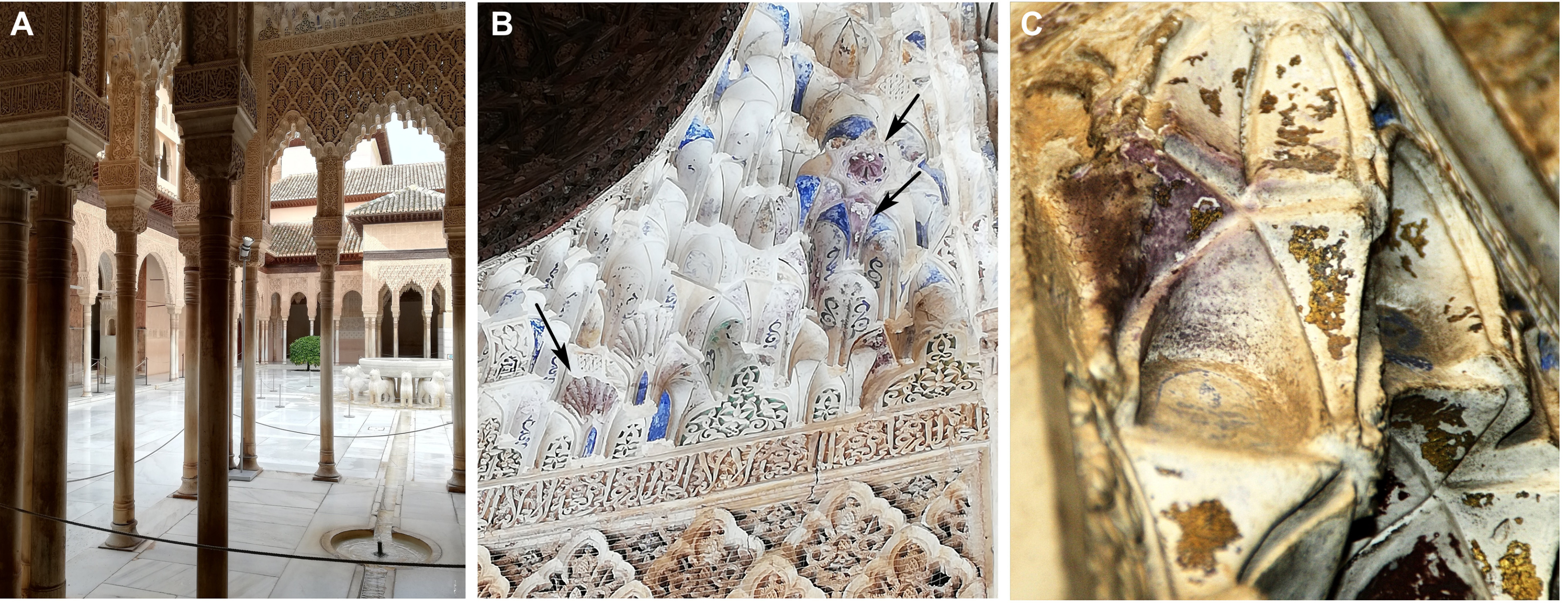
Parts of Alhambra’s ceilings are gilded with sheets of gold leaf, but in places, these have developed unusual purple blotches. Purple may have been associated with emperors since Roman times, but neither the rulers nor builders of the palace intended this effect. Instead, Professor Carolina Cardell and Dr Isabel Guerra of the University of Granada have explained in Science Advances, it arose from a string of chemical reactions that led to the gold becoming nanoparticles.
The patches puzzled observers because, as the paper notes, “pure gold (Au) is the least reactive metal in natural and industrial environments. Au does not discolor under sunlight or alter under common environmental situations including humidity, air pollution, corrosive gasses, and high temperatures.”
Although Alhambra dates from what is sometimes known as Spain’s Islamic golden age, the ornaments were not solid gold – that would be both impractical and ridiculously expensive. It also would probably never have survived centuries of thieves. Instead, they are made of tin gilded with a thin golden layer.
(A) General view of the Lions palace at Alhambra. (B) Polychrome remains exhibiting traces of purple color at confined wet sites (black arrows). (C) Damaged gilded tin with areas tinted purple in the muqarnas. Image Credit: Cardell and Guerra/Science Advances
A 2006 paper described the purple color, noting it only occurred in moist areas, but was unable to explain the cause.
Cardell and Guerra used a high-resolution field-emission scanning electron microscope to study the purple patches, and compare them with areas that had retained their original golden hue.
The color comes from the fact that gold particles, like those of other metals, have different properties when just nanometers wide compared to bulk materials. In fact, one can get most of the rainbow from gold particles suspended in water just by changing their size and shape. Being similar in size to the wavelengths of visible light, the particles absorb certain photons, reflecting those longer and shorter to be collected by our eyes.
(D) The gilded tin structure showing from inside to outside: corroded gray-black metallic foil, damaged metallic golden leaf (layer 2); fragments of iridescent purple-grayish covering and purple-tinted whitish coat at surface. (E) Polarized light microscope image of the gilding cross section. Note the irregular surface of the gray metallic foil (layer 1) and the crater-shaped voids in the gilded tin (circles). Image credit: C. Cardell and I. Guerra, University of Granada, Spain.
The authors found exposure to chlorine-rich water had broken the gilding down into nanoparticles around 70 nanometers wide – the right size to reflect the violet part of the spectrum while absorbing most other light we can see. The effect was deemed unattractive in the 19th century, and covered with a gypsum coat but some areas are starting to show through.
Besides the presence of water, the breakdown of gold to nanoparticle size depends on how porous the gold leaf is, and its adhesion to the underlying tin. The presence of tiny fissures can trigger galvanic corrosion, with the tin acting as the anode and becoming oxidized. Electrons released by the tin move to the gold, which acts as an inert electrode and therefore doesn’t react.
Thanks to pollution, however, the gold has become partially covered in grime. This creates variations in oxygen concentration on the surface of the gold, with oxygen-deficient areas dissolving into microflakes. The fact the gilders mixed some silver in with the gold enhanced the process.
Many reducing agents could have produced spherical gold nanoparticles from the microflakes, but the authors think the most likely are tin ions in chloride solutions. The chlorine ions that started the process are also probably a product of air pollution, rather than salt blown in from the sea.
Besides solving an old mystery, the authors hope their work will help conservators prevent corrosion of other previous historical sites.
Source Link: Why Did This Famous Medieval Palace’s Golden Ceilings Start Turning Purple?