Researchers have been able to trap electrons in a three-dimensional crystal for the first time, a breakthrough that allowed them to start toying with the quantum effects that the electrons produce when in a state called an electronic “flat band” – and that includes superconductivity.
An electron moving through a 3D material would interact with the lattice of its atoms in different way, so the electron’s kinetic energy is usually defined in terms of a range or band. If the electron’s band is flat, it means that the range is zero – its energy is independent of the interaction with the lattice. Simplistically, that electron has zero velocity and gets stuck around a particular location.
When an electron is in the flat band, it is still interacting with the electrons of the atoms around its location. These interactions have too little energy to be anything but negligible when an electron is moving to the material, but when the electron is stuck in place, suddenly they matter. And peculiar quantum properties become apparent such as superconductivity and other interesting electromagnetic properties.
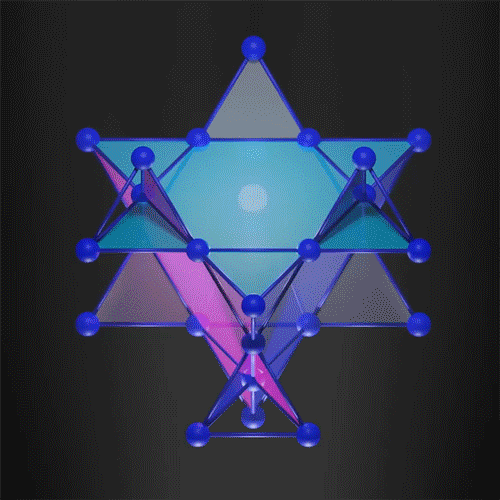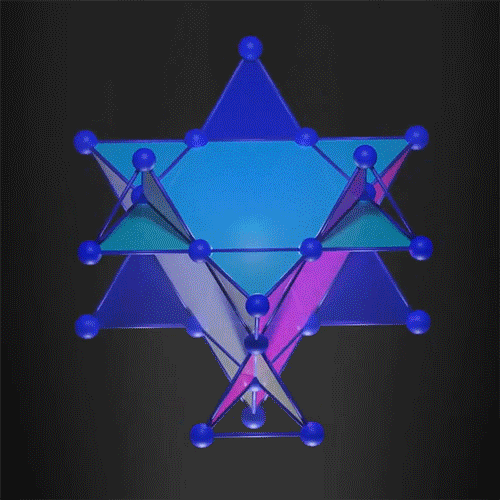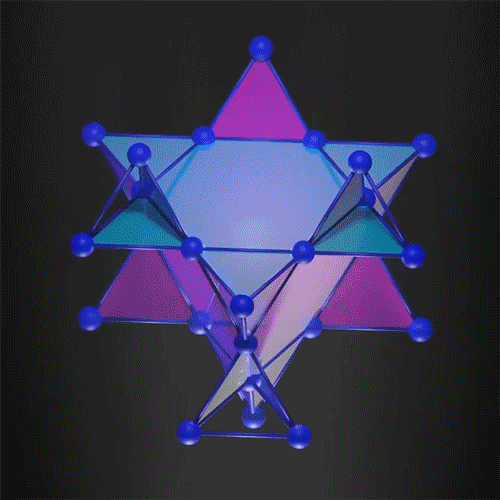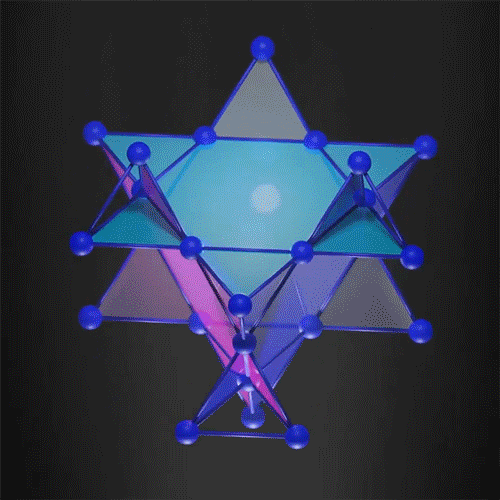
The kagome 3D lattice can trap the electrons.
Image Credit: courtesy of the researchers via MIT News
In the new work, researchers demonstrated that it is possible to create a 3D flat band, trapping the electron in all three dimensions. They used a 3D kagome-shaped lattice, which is used in the traditional Japanese art of basket weaving. Similar 2D lattices already demonstrated flat band electrons so the team felt this was a way to successfully create one in 3D.
“Now that we know we can make a flat band from this geometry, we have a big motivation to study other structures that might have other new physics that could be a platform for new technologies,” study author Joseph Checkelsky, associate professor of physics at MIT, said in a statement.
By making a chemical modification, the system was turned into a superconductor. This is a material through which electrons flow without resistance. To make the crystal the team synthesized pyrochlore crystals in the lab.
“It’s not dissimilar to how nature makes crystals,” Checkelsky explained. “We put certain elements together — in this case, calcium and nickel — melt them at very high temperatures, cool them down, and the atoms on their own will arrange into this crystalline, kagome-like configuration.”
Switching in atoms of rhodium and ruthenium instead of nickel creates the same geometrical configuration but pushes the value of the flat band to zero energy (not just zero velocity) – that’s where superconductivity happens.
“This presents a new paradigm to think about how to find new and interesting quantum materials,” added co-author professor of physics Riccardo Comin. “We showed that, with this special ingredient of this atomic arrangement that can trap electrons, we always find these flat bands. It’s not just a lucky strike. From this point on, the challenge is to optimize to achieve the promise of flat-band materials, potentially to sustain superconductivity at higher temperatures.”
These crystals or others like them might one day be optimized to build ultra-efficient power lines, create powerful quantum computers, and even faster electronic devices.
The study is published in the journal Nature.
Source Link: Electrons Trapped In A 3D Crystal For Very First Time